Abstract
Polyimide (PI) and polydimethylsiloxane (PDMS) are widely used materials in biomedical sensor development. The hydrophobic property of PDMS makes it difficult to bind with other materials, such as PI, which is commonly used in sensor applications. This paper employs the chemical functionalization of the PDMS and PI surfaces via epoxy-thiol click chemistry to achieve irreversible bonding. The bonding strength between the PDMS and PI is tested using a peel-off test method where adhesive and cohesive failures are observed. To demonstrate the importance of strong bonding, a wireless pressure sensor is developed. The sensor is tested for cyclic pressures over 1 million cycles with no evidence of bonding failures. This irreversible bonding can improve sensor integrity, reliability, and stability, especially for biomedical applications.
1. Introduction
Polyimides are one of the common materials used in biomedical devices due to their flexible nature and stability during harsh fabrication conditions. Flexible electronics such as antennas, light-emitting diodes, and organic transistors are fabricated on polyimide substrates [1]. The existing in vitro studies have shown that numerous polyimides are nontoxic and biocompatible and show a minor change in their properties over a 20-month-long period during incubation in physiological conditions [2]. Due to their biocompatible properties, polyimides are widely used in strain sensors, pressure sensors [3], and wearable electronics in biomedical applications [4].
Mostly, polyimides are flexible, but they cannot be stretched or compressed. Therefore, elastomers are used in combination with polyimides to make the device compressible. Styrene-Butadiene-Styrene (SBS), polydimethylsiloxane (PDMS), and rubber are the most commonly used elastomers. Due to the weak adhesion of elastomers with the metal, it is difficult to fabricate electrode patterns on the surface of the elastomer directly [5]. Therefore, more complex fabrication techniques are used to fabricate the electrode directly on the elastomer surface; however, these techniques have limitations in terms of reliability, resolution, stability, performance, and long-term integrity of the metallic traces [6]. As a result, electrode patterns are manufactured on a thin layer of the extra substrate rather than directly fabricating metallic electrodes on the elastomers.
Due to the hydrophobic nature of PDMS, the bonding between PDMS and PI is challenging; hence, a chemical technique for surface modification has been proposed to improve the bonding between PDMS and PI. However, this technique needs a specialized vacuum deposition system which increases the production cost [7]. The epoxy-thiol click chemistry is a very effective and reliable method for bonding PDMS and PI substrates under fairly mild circumstances [5].
This study has developed a pressure sensor utilizing PDMS and a copper-coated PI sheet to highlight the significance of irreversible bonding achieved between PDMS and PI using epoxy-thiol click chemistry. This pressure sensor was reported in one of our prior studies [3], where the sensor patterns were achieved using a cost-effective wet etching technique. To chemically functionalize the surfaces, plasma-treated prefabricated PDMS sheets and electrode-patterned PI sheets were immersed in (3-mercaptopropyl) trimethoxysilane (MPTMS) and (3-glycidyloxypropyl) trimethoxysilane (GPTMS), respectively. The final assembly was created after chemical treatment, and the sensor was then placed under pressure for 24 h at room temperature. Later, a peel-off test was carried out to gauge the bonding strength, and sensor performance was shown.
2. Materials and Methods
2.1. Sensor Designing
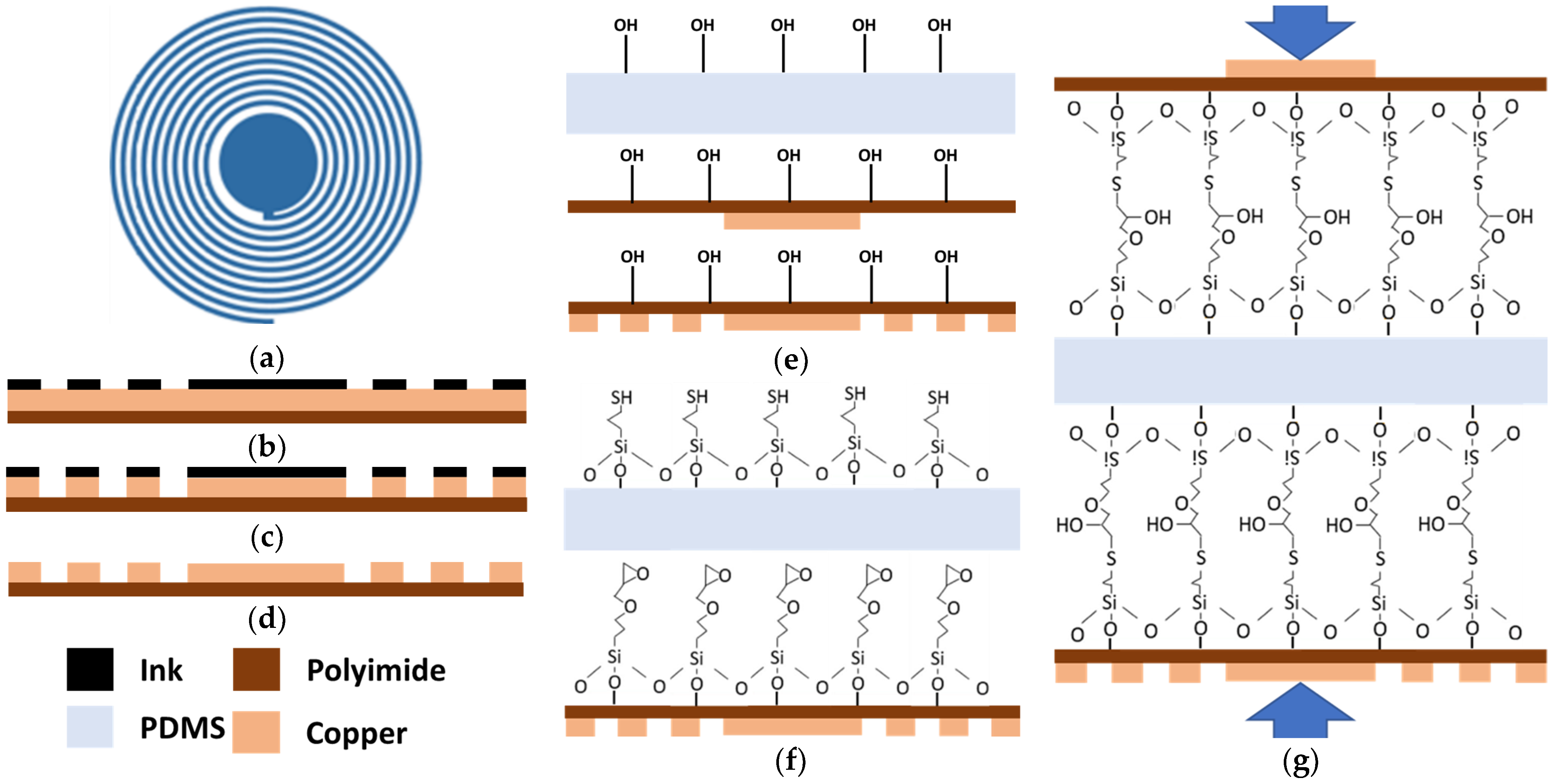
A wireless pressure sensor based on an LC resonance principle was developed using a cost-effective and accessible fabrication technique. A circular-shaped sensor was designed in AutoCAD 2020 and the geometry of the sensor is shown in Figure 1a. The central solid disk represents the capacitor while the spiral traces represent the inductance of the sensor. The sensor resonates at the resonance frequency , which changes with the change in the pressure. The resonance frequency can be calculated as:
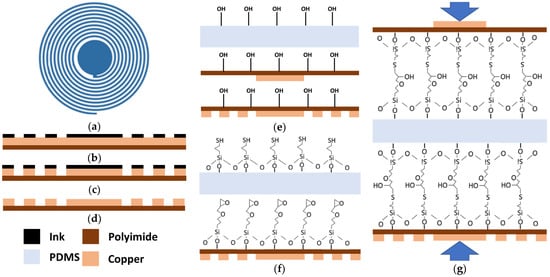
Figure 1.
Sensor designing and fabrication process: (a) Pressure sensor geometry, (b) Ink mask printed on copper-coated polyimide sheet, (c) Sensor patterns after etching process, (d) Sensor pattern after cleaning with acetone, (e) Plasma treatment of PDMS and polyimide, (f) Chemical surface functionalization, (g) PDMS sandwiched between polyimide sheets to make the final assembly of the sensor under pressure.
2.2. Sensor Fabrication
2.2.1. Sensor Pattern Development
Figure 1 shows the different stages of the fabrication process. Firstly, the mask of the sensor was directly printed on a 50 μm thick copper-coated polyimide film (Flexible isolating circuit 50 μm-coppered 35 μm-1 sided, CIF, Buc, France) with a LaserJet printer (HP M553, HP Technology, Dublin, Ireland) (Figure 1b). In the next stage, the printed copper sheet was attached to the PCB holder of the bubble etching tank. To remove all the unwanted copper, sodium persulphate is used as an etchant in the bubble tank at 45 °C as shown in Figure 1c. Acetone and hot water were used to remove the ink and residual etchant from the patterned electrodes on the polyimide substrate (Figure 1d). The conductivity of the inductive traces was tested using the short circuit method from a digital multimeter (DMM). These traces were also analyzed under the digital magnifier.
2.2.2. Plasma Treatment of PDMS and Polyimide
A 200 μm thick layer of PDMS was cut to the sensor’s dimensions. The PDMS and sensor conductive pattern on the flexible polyimide sheets were placed inside the glass chamber of the plasma oven. The glass chamber of the plasma oven was pressurized to a 100–130 mTorr pressure using an oil-free dry oxygen pump (PDC-OPD-2). The RF level was raised to high for a 45-s plasma treatment on the PDMS and polyimide surfaces after reaching the 100–130 mTorr pressure. The plasma treatment was performed to achieve the hydroxylation on the surface of PDMS and patterned polyimide sheet as shown in Figure 1e.
2.2.3. Chemical Solution Preparation and Surface Functionalization
For the chemical surface activation, (3-glycidyloxypropyl) trimethoxysilane (GPTMS, 98%, PRODUCT# 440167) and (3-mercaptopropyl) trimethoxysilane (MPTMS, 95%, PRODUCT #175617) sourced from Sigma-Aldrich were used. There were prepared from 1% (v/v) solutions of GPTMS in the methanol and MPTMS in the methanol in the lab under the nitrogen gas environment. In an alternative version of sensor fabrication, 2% (v/v) solutions of GPTMS in methanol, and MPTMS in methanol were prepared for the chemical surface activation.
The plasma-treated PDMS and patterned polyimide sheets were placed inside the 1% (v/v) solution of MPTMS in methanol and GPTMS in methanol for 1 h to achieve the functionalized surfaces of PDMS and Polyimide as shown in Figure 1f. After 1 h liquid deposition, these chemically treated PDMS and polyimide sheets were washed in deionized water and dried at room temperature.
For an alternative fabrication of the sensor to test the effect of solution strength on bonding, these plasma-treated PDMS, and polyimide sheets were immersed in the 2% (v/v) solution of MPTMS in methanol and 2% (v/v) solution of GPTMS in methanol, respectively.
2.2.4. Final Assembling of Sensor
Later, this chemically functionalized PDMS thin sheet was sandwiched between the patterned Polyimide sheet and kept under uniform pressure of 10–15 kPa for 24 h to achieve the irreversible bonding between the PDMS and patterned Polyimide as shown in Figure 1g. This irreversible bonding between PDMS and Polyimide surfaces increases the integrity of the pressure sensor.
3. Results and Discussion
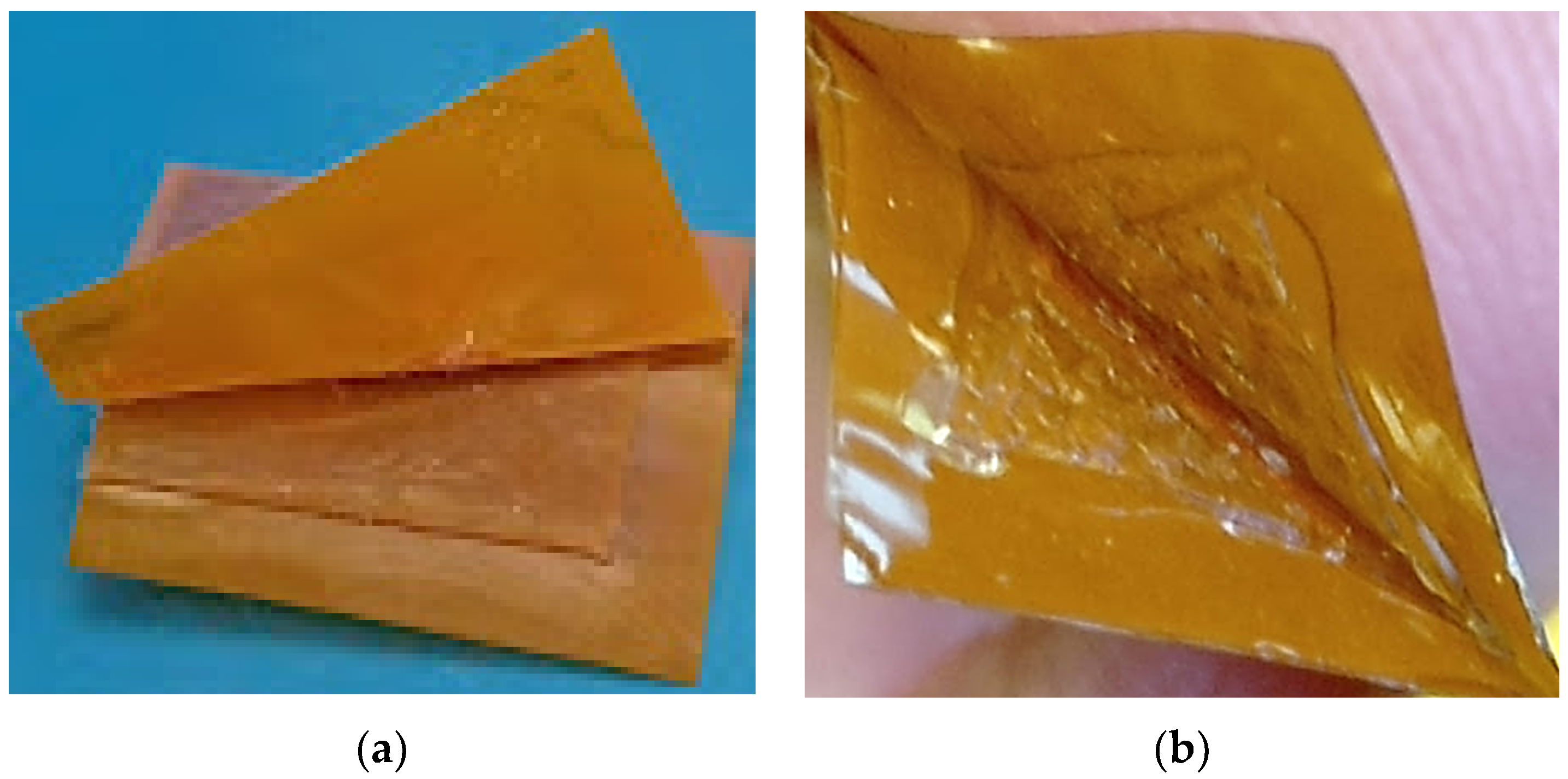
This study used a hand-force peel test method to determine the degree of adhesion between the PDMS and conductive patterned polyimide. During the peel-off test, it was observed that the bonding between the polyimide surface and PDMS is not irreversible throughout the sensor area when 1% (v/v) solution of MPTMS in methanol and 1% (v/v) solution of GPTMS in methanol were used for chemically surface activation. An adhesive failure (surficial bonding between the two different layers of materials fails) was observed between the PDMS and polyimide-bonded surfaces as shown in Figure 2a. As a next step, these plasma-treated PDMS and polyimide sheets were immersed in the 2% (v/v) solution of MPTMS in methanol and 2% (v/v) solution of GPTMS in methanol, respectively. During the peel-off test, a cohesive failure (when one of the bonded materials tear-off instead of surficial bonding failure) was observed instead of adhesive failure as shown in Figure 2b.
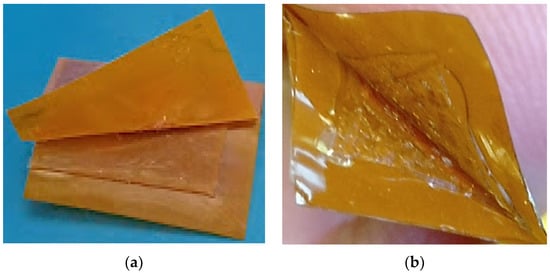
Figure 2.
Peel-off test (a) Adhesive failure between PDMS and Polyimide (b) Cohesive failure of PDMS proofing irreversible bonding between polyimide and PDMS.
To simulate fatigue testing, the fabricated sensor, the fabricated sensor was placed inside the air pressure chamber and cyclic pressure 45–95 mmHg was applied for more than 1 million cycles. However, after 1 million pressure cycles we have seen a cohesive failure which means that the bonding between PDMS and Polyimide was irreversible using this epoxy-thiol click chemistry technique.
4. Conclusions
This study has presented an efficient and cost-effective methodology (epoxy-thiol click chemistry) to functionalize the Polyimide and PDMS surfaces to achieve an irreversible bonding. Moreover, a wireless pressure sensor was developed using chemically functionalized bonding. The bonding strength was tested using the peel-off test method. An adhesive failure was observed between PDMS and Polyimide for 1% (v/v); however, the 2% (v/v) resulted in a cohesive failure (excellent adhesion) of PDMS, which shows the bonding between Polyimide and PDMS was irreversible. Moreover, it was observed that the bonding did not fail over the sensor’s 1,000,000 cycles of pressure testing, indicating that the sensor’s integrity, dependability, and stability had all improved. Future optimization of this epoxy-thiol click chemistry is possible to improve the bonding of various sensor materials.
Author Contributions
Conceptualization, M.F.; methodology, M.F.; validation, M.F. and B.A.; formal analysis, M.F.; resources, W.W.; writing—original draft preparation, M.F. and B.A.; writing—review and editing, W.W., A.S. and A.E.; visualization, M.F.; supervision, W.W. and A.S.; project administration, A.S.; funding acquisition, W.W. and A.S. All authors have read and agreed to the published version of the manuscript.
Funding
The research leading to this publication was funded by the Science Foundation Ireland Research Professorship Award (grant no. 15/RP/2765) and the Government of Ireland Disruptive Technology Innovation Fund (grant no. DT20180031A).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Khaleel, H.R.; Al-Rizzo, H.M.; Rucker, D.G.; Mohan, S. A compact polyimide-based UWB antenna for flexible electronics. IEEE Antennas Wirel. Propag. Lett. 2012, 11, 564–567. [Google Scholar] [CrossRef]
- Richardson, R.R., Jr.; Miller, J.A.; Reichert, W.M. Polyimides as biomaterials: Preliminary biocompatibility testing. Biomaterials 1993, 14, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Iqbal, T.; Vazquez, P.; Farid, N.; Thampi, S.; Wijns, W.; Shahzad, A. Thin-film flexible wireless pressure sensor for continuous pressure monitoring in medical applications. Sensors 2020, 20, 6653. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-H.; Baek, D.H.; Choi, Y.Y.; Lee, K.H.; Kim, H.C.; Lee, S.-H. Wearable polyimide--PDMS electrodes for intrabody communication. J. Micromech. Microeng. 2010, 20, 25032. [Google Scholar] [CrossRef]
- Hoang, M.V.; Chung, H.-J.; Elias, A.L. Irreversible bonding of polyimide and polydimethylsiloxane (PDMS) based on a thiol-epoxy click reaction. J. Micromech. Microeng. 2016, 26, 105019. [Google Scholar] [CrossRef]
- Agostini, M.; Greco, G.; Cecchini, M. Polydimethylsiloxane (PDMS) irreversible bonding to untreated plastics and metals for microfluidics applications. APL Mater. 2019, 7, 81108. [Google Scholar] [CrossRef]
- Kim, D.H.; Song, J.; Choi, W.M.; Kim, H.S.; Kim, R.H.; Liu, Z.; Huang, Y.Y.; Hwang, K.C.; Zhang, Y.W.; Rogers, J.A. Materials and noncoplanar mesh designs for integrated circuits with linear elastic responses to extreme mechanical deformations. Proc. Natl. Acad. Sci. USA 2008, 105, 18675–18680. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).