Early Detection of Volatile Tumor Biomarkers Using Chemoresistive Sensors and MEMS-Based Preconcentration: A Study on K562 Cell Line †
Abstract
1. Introduction
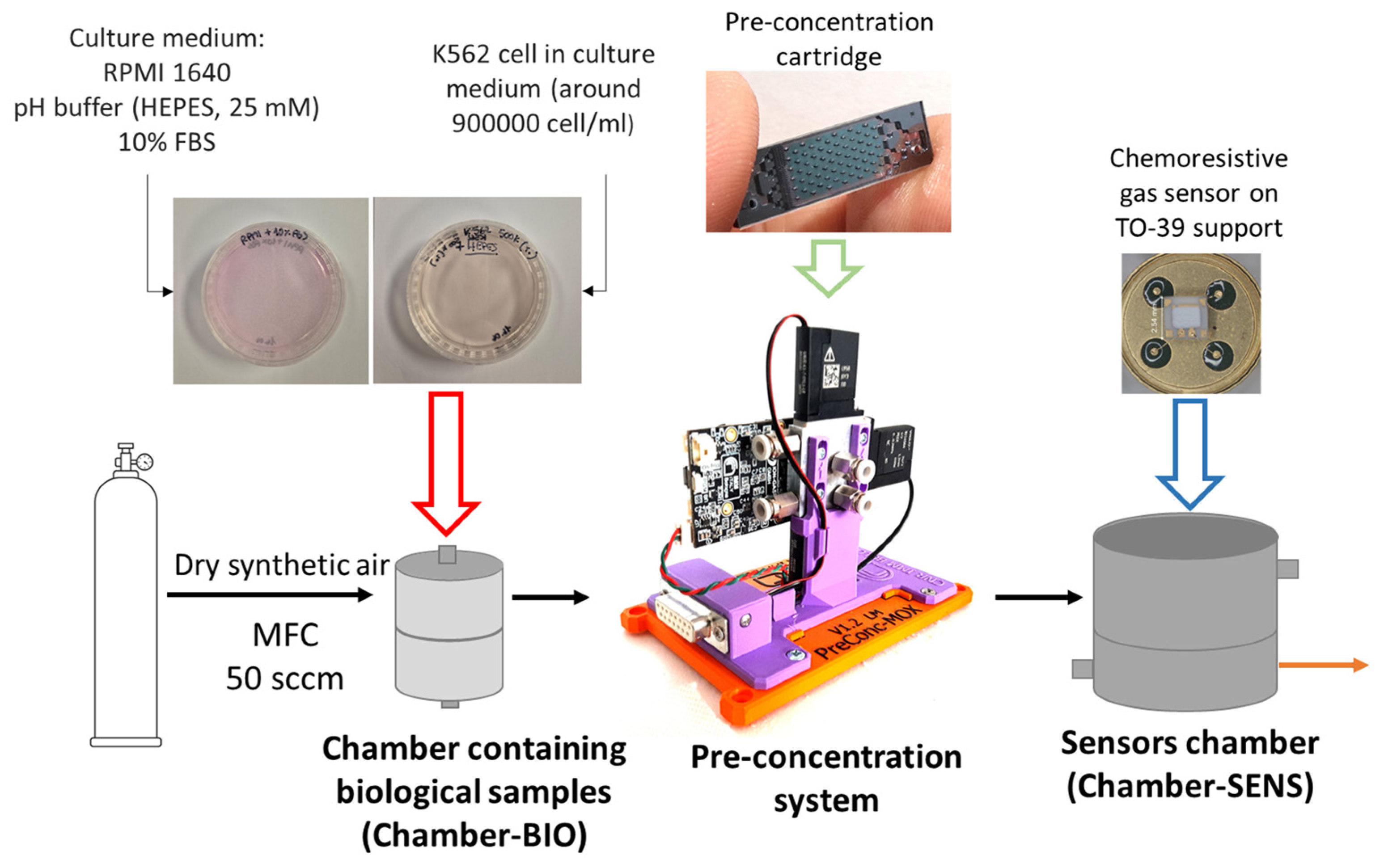
2. Materials and Methods
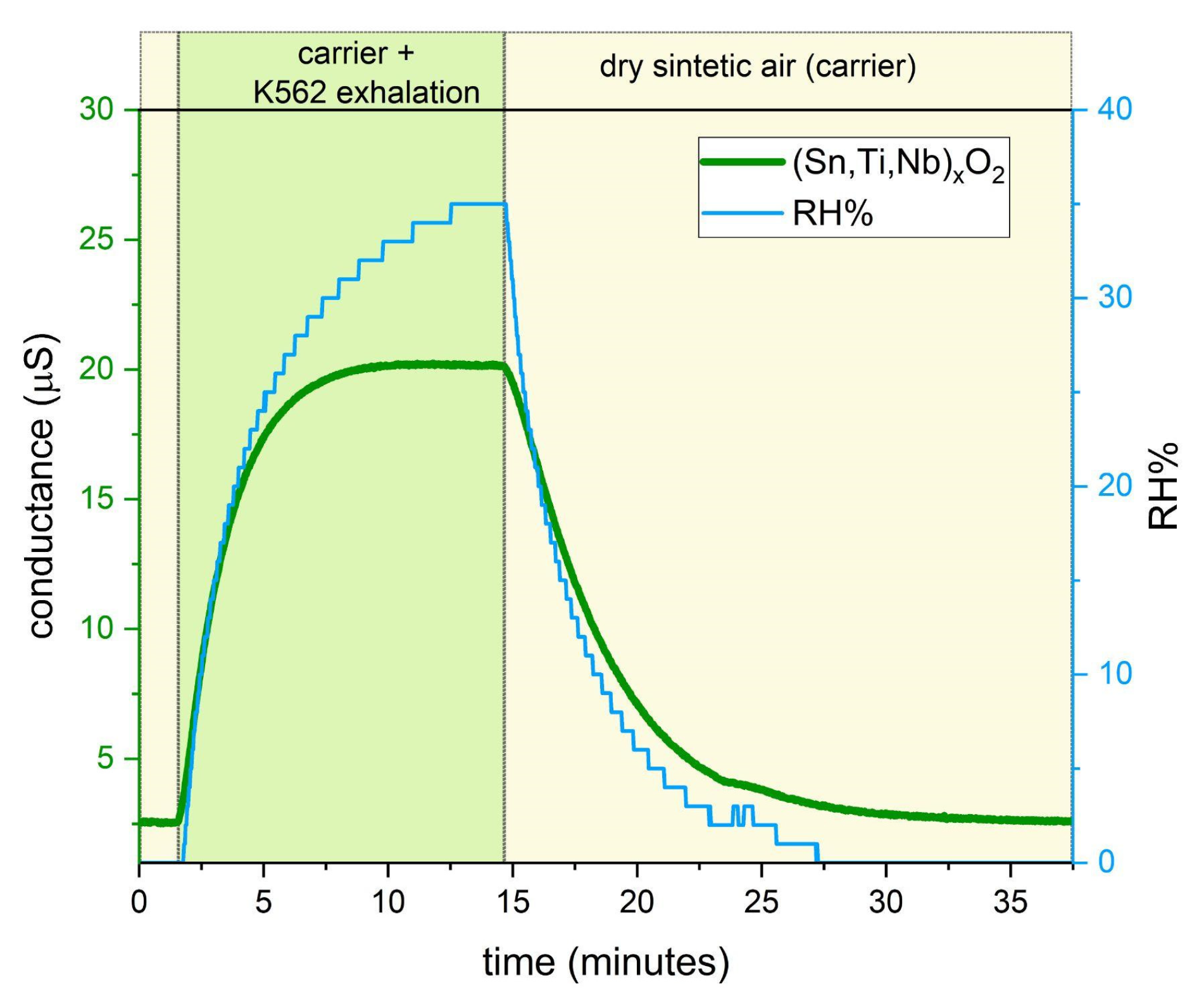
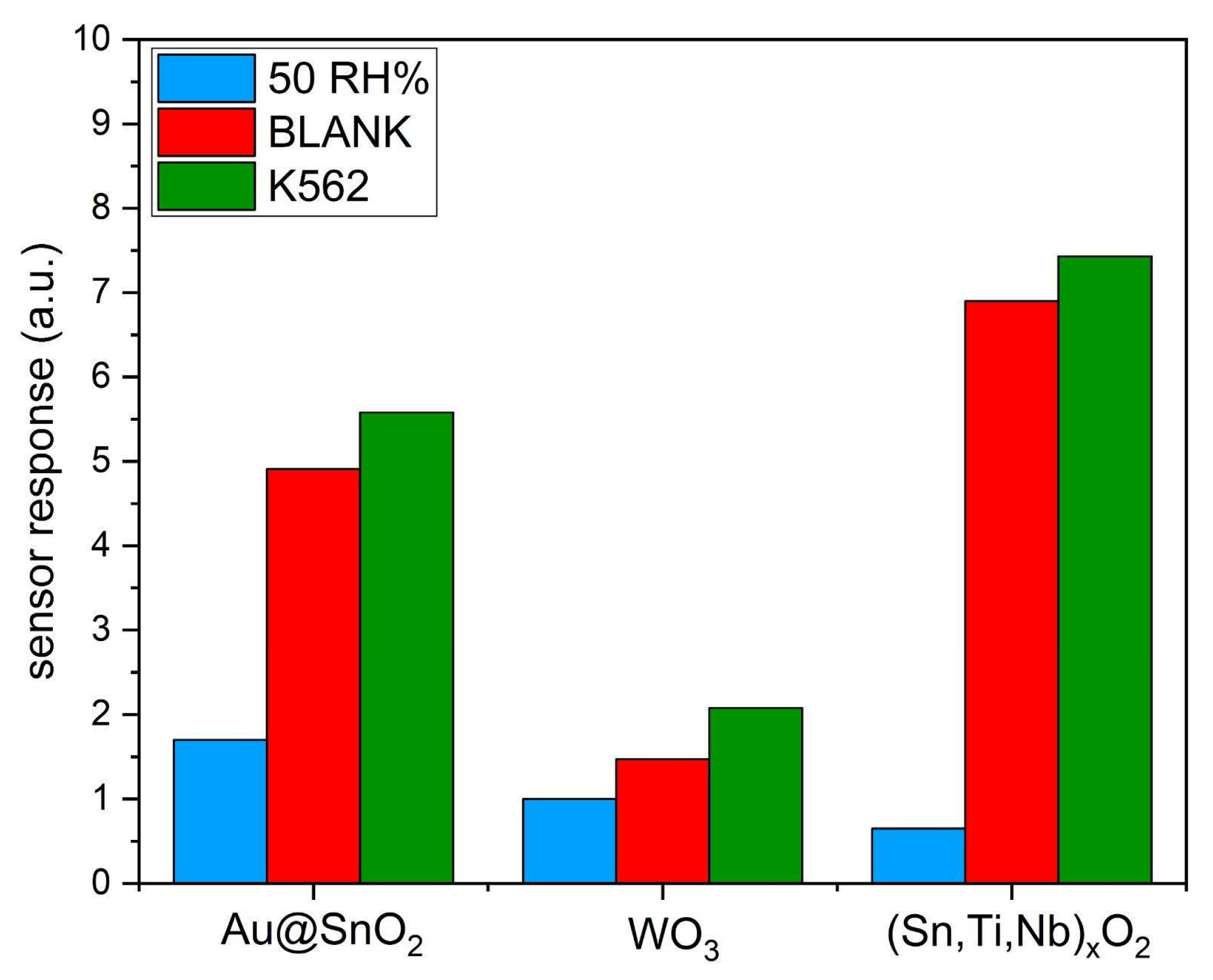
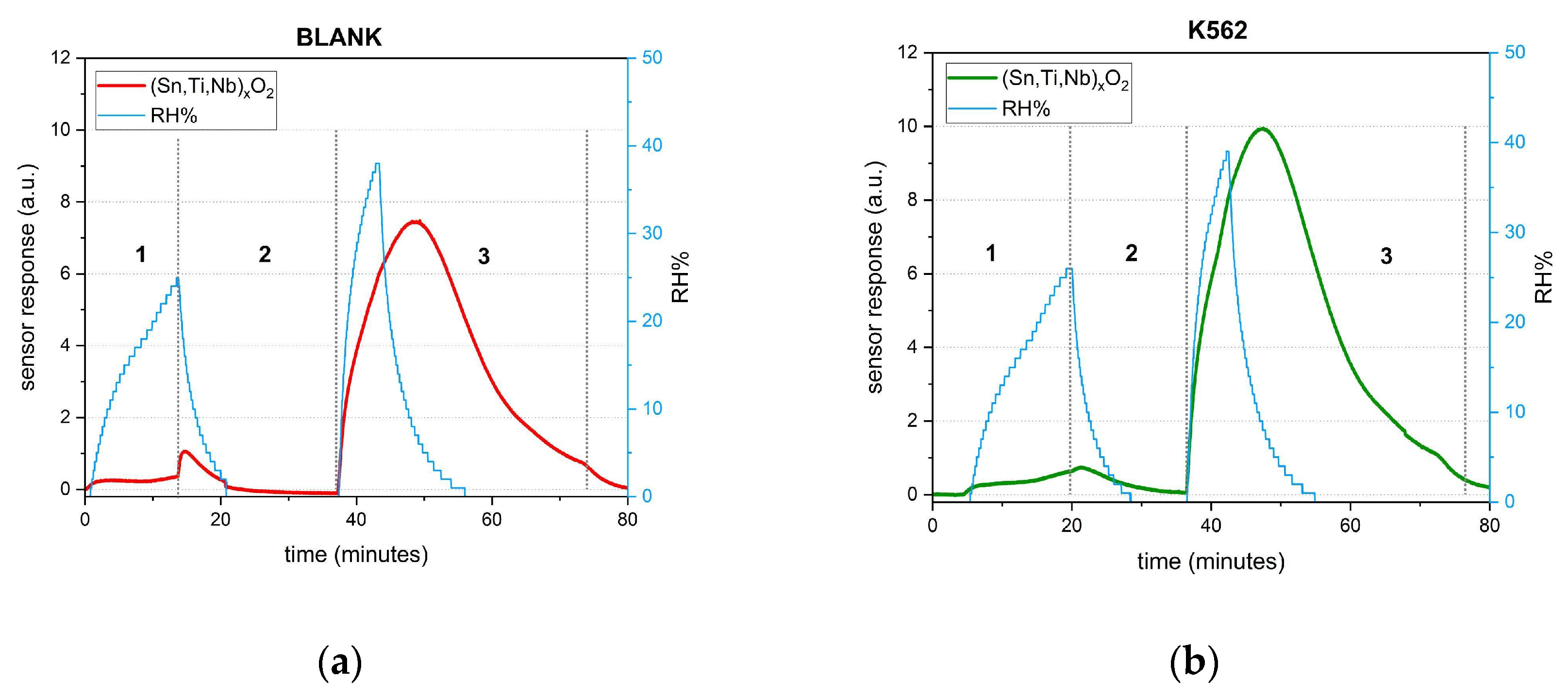
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VOCs | Volatile Organic Compounds |
| MOXs | Metal Oxides |
| MEMSs | Micro-Electro-Mechanical Systems |
| RH | Relative Humidity |
References
- Gonçalves, A.C.; Alves, R.; Sarmento-Ribeiro, A.B. Advancements in Biomarkers and Molecular Targets in Hematological Neoplasias. Int. J. Mol. Sci. 2024, 25, 6570. [Google Scholar] [CrossRef] [PubMed]
- Furuhashi, T.; Toda, K.; Weckwerth, W. Review of Cancer Cell Volatile Organic Compounds: Their Metabolism and Evolution. Front. Mol. Biosci. 2025, 11, 1499104. [Google Scholar] [CrossRef]
- Hakim, M.; Broza, Y.Y.; Barash, O.; Peled, N.; Phillips, M.; Amann, A.; Haick, H. Volatile Organic Compounds of Lung Cancer and Possible Biochemical Pathways. Chem. Rev. 2012, 112, 5949–5966. [Google Scholar] [CrossRef]
- Filipiak, W.; Mochalski, P.; Filipiak, A.; Ager, C.; Cumeras, R.; E. Davis, C.; Agapiou, A.; Unterkofler, K.; Troppmair, J. A Compendium of Volatile Organic Compounds (VOCs) Released By Human Cell Lines. Curr. Med. Chem. 2016, 23, 2112–2131. [Google Scholar] [CrossRef]
- Amoêdo, N.D.; Valencia, J.P.; Rodrigues, M.F.; Galina, A.; Rumjanek, F.D. How Does the Metabolism of Tumour Cells Differ from That of Normal Cells. Biosci. Rep. 2013, 33, e00080. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg Effect: The Metabolic Requirements of Cell Proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef] [PubMed]
- Rath, R.J.; Farajikhah, S.; Oveissi, F.; Dehghani, F.; Naficy, S. Chemiresistive Sensor Arrays for Gas/Volatile Organic Compounds Monitoring: A Review. Adv. Eng. Mater. 2023, 25, 2200830. [Google Scholar] [CrossRef]
- Schmitt, E.A.; Krott, M.; Epifani, M.; Suematsu, K.; Weimar, U.; Barsan, N. Volatile Organic Compound Sensing with WO3 -Based Gas Sensors: Surface Chemistry Basics. J. Phys. Chem. C 2024, 128, 1633–1643. [Google Scholar] [CrossRef]
- Tomić, M.; Šetka, M.; Vojkůvka, L.; Vallejos, S. VOCs Sensing by Metal Oxides, Conductive Polymers, and Carbon-Based Materials. Nanomaterials 2021, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Pecunia, V.; Petti, L.; Andrews, J.B.; Ollearo, R.; Gelinck, G.H.; Nasrollahi, B.; Jailani, J.M.; Li, N.; Kim, J.H.; Ng, T.N.; et al. Roadmap on Printable Electronic Materials for Next-Generation Sensors. Nano Futur. 2024, 8, 032001. [Google Scholar] [CrossRef]
- H S, S.; Vijayan, A.; P, P.; Rao, A. Scalable, Sensitive, Smart: The Role of Inkjet Printing in next-Generation Chemiresistive Gas Sensors. Mater. Res. Express 2025, 12, 092001. [Google Scholar] [CrossRef]
- Neri, G. First Fifty Years of Chemoresistive Gas Sensors. Chemosensors 2015, 3, 1–20. [Google Scholar] [CrossRef]
- Love, C.; Nazemi, H.; El-Masri, E.; Ambrose, K.; Freund, M.S.; Emadi, A. A Review on Advanced Sensing Materials for Agricultural Gas Sensors. Sensors 2021, 21, 3423. [Google Scholar] [CrossRef]
- Fabbri, B.; Valt, M.; Parretta, C.; Gherardi, S.; Gaiardo, A.; Malagù, C.; Mantovani, F.; Strati, V.; Guidi, V. Correlation of Gaseous Emissions to Water Stress in Tomato and Maize Crops: From Field to Laboratory and Back. Sens. Actuators B Chem. 2020, 303, 127227. [Google Scholar] [CrossRef]
- John, A.T.; Murugappan, K.; Nisbet, D.R.; Tricoli, A. An Outlook of Recent Advances in Chemiresistive Sensor-Based Electronic Nose Systems for Food Quality and Environmental Monitoring. Sensors 2021, 21, 2271. [Google Scholar] [CrossRef]
- Rodríguez-Aguilar, M.; Díaz de León-Martínez, L.; Gorocica-Rosete, P.; Pérez-Padilla, R.; Domínguez-Reyes, C.A.; Tenorio-Torres, J.A.; Ornelas-Rebolledo, O.; Mehta, G.; Zamora-Mendoza, B.N.; Flores-Ramírez, R. Application of Chemoresistive Gas Sensors and Chemometric Analysis to Differentiate the Fingerprints of Global Volatile Organic Compounds from Diseases. Preliminary Results of COPD, Lung Cancer and Breast Cancer. Clin. Chim. Acta 2021, 518, 83–92. [Google Scholar] [CrossRef]
- Nasiri, N.; Clarke, C. Nanostructured Chemiresistive Gas Sensors for Medical Applications. Sensors 2019, 19, 462. [Google Scholar] [CrossRef]
- Güntner, A.T.; Abegg, S.; Königstein, K.; Gerber, P.A.; Schmidt-Trucksäss, A.; Pratsinis, S.E. Breath Sensors for Health Monitoring. ACS Sens. 2019, 4, 268–280. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Zhang, W.; Yang, J.; Pei, Y.; Tan, X.; Dong, B.; Song, H.; Xu, L. Chemiresistive Gas Sensors for Intelligent Sensing: Design Strategies, Emerging Applications and Future Challenges. Chem. Soc. Rev. 2025, 54, 11302–11367. [Google Scholar] [CrossRef]
- Moon, H.G.; Jung, Y.; Han, S.D.; Shim, Y.-S.; Shin, B.; Lee, T.; Kim, J.-S.; Lee, S.; Jun, S.C.; Park, H.-H.; et al. Chemiresistive Electronic Nose toward Detection of Biomarkers in Exhaled Breath. ACS Appl. Mater. Interfaces 2016, 8, 20969–20976. [Google Scholar] [CrossRef] [PubMed]
- Sachan, A.; Castro, M.; Feller, J.-F. Volatolomics for Anticipated Diagnosis of Cancers with Chemoresistive Vapour Sensors: A Review. Chemosensors 2025, 13, 15. [Google Scholar] [CrossRef]
- Astolfi, M.; Rispoli, G.; Benedusi, M.; Zonta, G.; Landini, N.; Valacchi, G.; Malagù, C. Chemoresistive Sensors for Cellular Type Discrimination Based on Their Exhalations. Nanomaterials 2022, 12, 1111. [Google Scholar] [CrossRef]
- Ortega, P.P.; Gherardi, S.; Spagnoli, E.; Fabbri, B.; Landini, N.; Malagù, C.; Macchi, C.; Aldao, C.M.; Ponce, M.A.; Simões, A.Z.; et al. Deciphering the CO Sensing Mechanisms of CeO2-Based Nanostructured Semiconductors: Influence of Doping, Morphology, and Humidity. Sens. Actuators B Chem. 2025, 440, 137921. [Google Scholar] [CrossRef]
- Spagnoli, E.; Valt, M.; Gaiardo, A.; Fabbri, B.; Guidi, V. Insights into the Sensing Mechanism of a Metal-Oxide Solid Solution via Operando Diffuse Reflectance Infrared Fourier Transform Spectroscopy. Nanomaterials 2023, 13, 2708. [Google Scholar] [CrossRef]
- Degler, D.; Junker, B.; Allmendinger, F.; Weimar, U.; Barsan, N. Investigations on the Temperature-Dependent Interaction of Water Vapor with Tin Dioxide and Its Implications on Gas Sensing. ACS Sens. 2020, 5, 3207–3216. [Google Scholar] [CrossRef]
- Madou, M.J.; Morrison, S.R. Chemical Sensing with Solid State Devices; Elsevier: Amsterdam, The Netherlands, 1989; ISBN 9780124649651. [Google Scholar]
- Zampolli, S.; Elmi, I.; Mancarella, F.; Betti, P.; Dalcanale, E.; Cardinali, G.C.; Severi, M. Real-Time Monitoring of Sub-Ppb Concentrations of Aromatic Volatiles with a MEMS-Enabled Miniaturized Gas-Chromatograph. Sens. Actuators B Chem. 2009, 141, 322–328. [Google Scholar] [CrossRef]
- Gregis, G.; Sanchez, J.-B.; Bezverkhyy, I.; Guy, W.; Berger, F.; Fierro, V.; Bellat, J.-P.; Celzard, A. Detection and Quantification of Lung Cancer Biomarkers by a Micro-Analytical Device Using a Single Metal Oxide-Based Gas Sensor. Sens. Actuators B Chem. 2018, 255, 391–400. [Google Scholar] [CrossRef]
- Yoon, J.-W.; Lee, J.-H. Toward Breath Analysis on a Chip for Disease Diagnosis Using Semiconductor-Based Chemiresistors: Recent Progress and Future Perspectives. Lab Chip 2017, 17, 3537–3557. [Google Scholar] [CrossRef]
- Lozzio, C.B.; Lozzio, B.B. Human Chronic Myelogenous Leukemia Cell-Line with Positive Philadelphia Chromosome. Blood. 1975;45(3):321-334. Blood 2016, 128, 1995. [Google Scholar] [CrossRef]
- Capuano, R.; Talarico, R.; Spitalieri, P.; Roberto, P.; Giuseppe, N.; Sangiuolo, F.; Di Natale, C. GC/MS-Based Analysis of Volatile Metabolic Profile Along in Vitro Differentiation of Human Induced Pluripotent Stem Cells. Bio-Protocol 2017, 7, e2642. [Google Scholar] [CrossRef] [PubMed]
- Spagnoli, E.; Krik, S.; Fabbri, B.; Valt, M.; Ardit, M.; Gaiardo, A.; Vanzetti, L.; Della Ciana, M.; Cristino, V.; Vola, G.; et al. Development and Characterization of WO3 Nanoflakes for Selective Ethanol Sensing. Sens. Actuators B Chem. 2021, 347, 130593. [Google Scholar] [CrossRef]
- Rossi, A.; Spagnoli, E.; Tralli, F.; Marzocchi, M.; Guidi, V.; Fabbri, B. New Approach for the Detection of Sub-Ppm Limonene: An Investigation through Chemoresistive Metal-Oxide Semiconductors. Sensors 2023, 23, 6291. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, M.; Zonta, G.; Gherardi, S.; Malagù, C.; Vincenzi, D.; Rispoli, G. A Portable Device for I–V and Arrhenius Plots to Characterize Chemoresistive Gas Sensors: Test on SnO2-Based Sensors. Nanomaterials 2023, 13, 2549. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tamisari, M.; Spagnoli, E.; Altieri, M.T.; Astolfi, M.; Borgatti, M.; Breveglieri, G.; Elmi, I.; Guidi, V.; Masini, L.; Rossi, A.; et al. Early Detection of Volatile Tumor Biomarkers Using Chemoresistive Sensors and MEMS-Based Preconcentration: A Study on K562 Cell Line. Eng. Proc. 2025, 118, 70. https://doi.org/10.3390/ECSA-12-26565
Tamisari M, Spagnoli E, Altieri MT, Astolfi M, Borgatti M, Breveglieri G, Elmi I, Guidi V, Masini L, Rossi A, et al. Early Detection of Volatile Tumor Biomarkers Using Chemoresistive Sensors and MEMS-Based Preconcentration: A Study on K562 Cell Line. Engineering Proceedings. 2025; 118(1):70. https://doi.org/10.3390/ECSA-12-26565
Chicago/Turabian StyleTamisari, Melissa, Elena Spagnoli, Maria Teresa Altieri, Michele Astolfi, Monica Borgatti, Giulia Breveglieri, Ivan Elmi, Vincenzo Guidi, Luca Masini, Arianna Rossi, and et al. 2025. "Early Detection of Volatile Tumor Biomarkers Using Chemoresistive Sensors and MEMS-Based Preconcentration: A Study on K562 Cell Line" Engineering Proceedings 118, no. 1: 70. https://doi.org/10.3390/ECSA-12-26565
APA StyleTamisari, M., Spagnoli, E., Altieri, M. T., Astolfi, M., Borgatti, M., Breveglieri, G., Elmi, I., Guidi, V., Masini, L., Rossi, A., Sabbioni, G., Tavaglione, E., Zampolli, S., & Fabbri, B. (2025). Early Detection of Volatile Tumor Biomarkers Using Chemoresistive Sensors and MEMS-Based Preconcentration: A Study on K562 Cell Line. Engineering Proceedings, 118(1), 70. https://doi.org/10.3390/ECSA-12-26565









