Optical Chemosensory Studies of Novel Amphiphilic D-A-π-A Benzothiadiazoles for Cyanide Detection †
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Photophysical Characterization of Benzothiadiazole Derivatives 1–3
2.2. Experimental Procedure for the Chemosensory Studies of Benzothiadiazoles 2 and 3
3. Results and Discussion
3.1. Photophysical Characterization of Benzothiadiazole Derivatives 1–3
3.2. Chemosensory Studies of Benzothiadiazoles 2 and 3
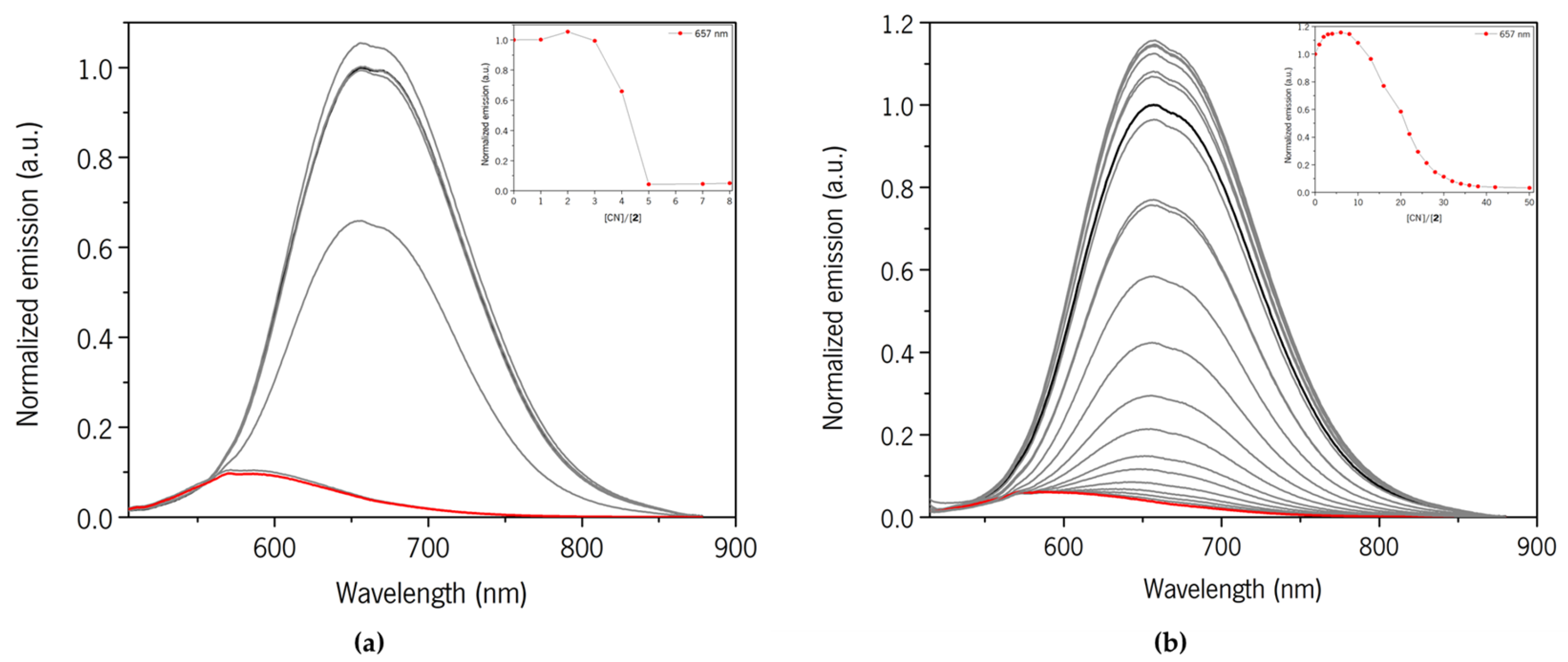
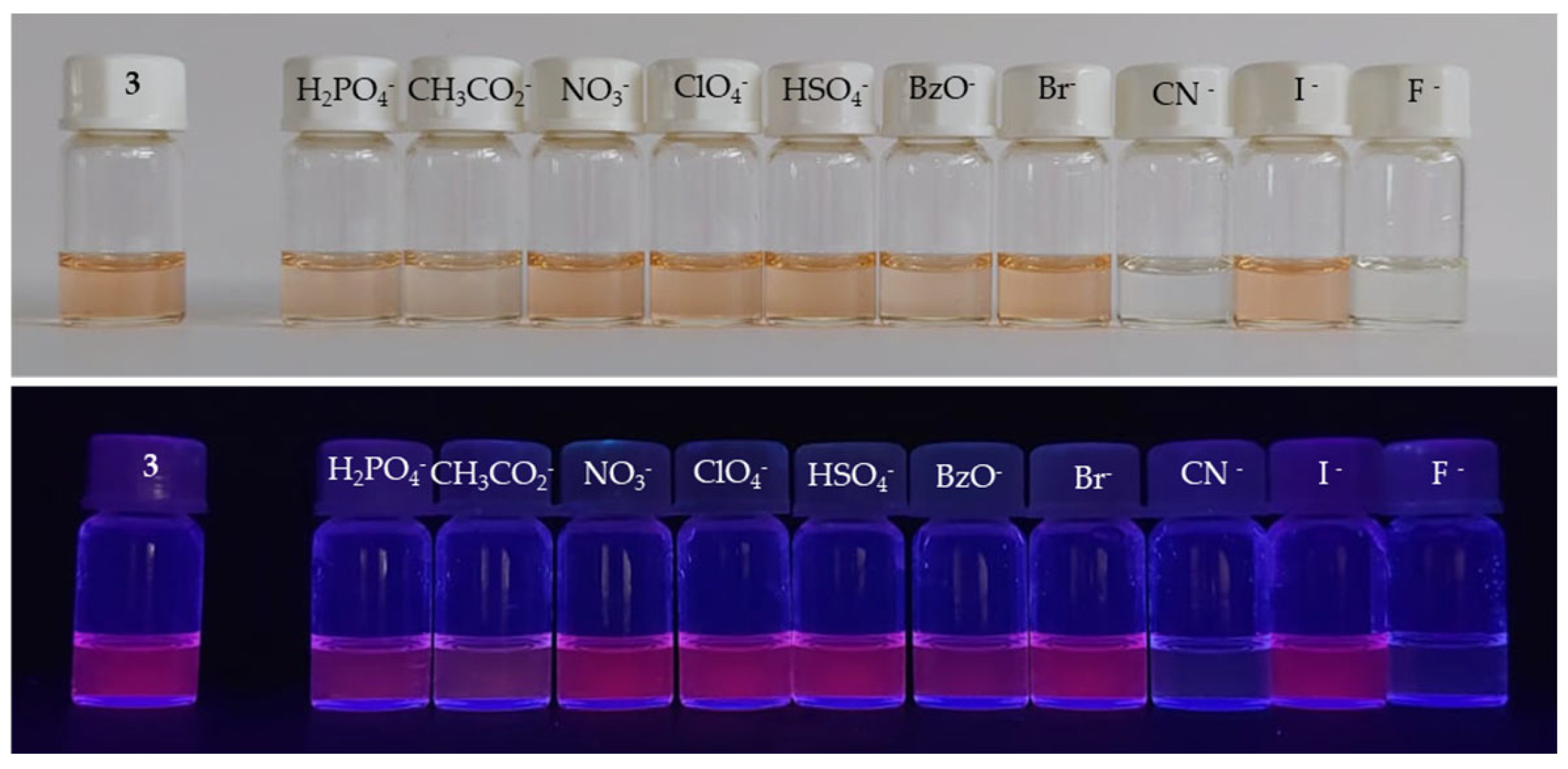
3.2.1. Compound 2
3.2.2. Compound 3
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chua, M.H.; Zhu, Q.; Tang, T.; Shah, K.W.; Xu, J. Diversity of Electron Acceptor Groups in Donor–Acceptor Type Electrochromic Conjugated Polymers. Sol. Energy Mater. Sol. Cells 2019, 197, 32–75. [Google Scholar] [CrossRef]
- Ersoy, G.; Henary, M. Roadmap for Designing Donor-π-Acceptor Fluorophores in UV-Vis and NIR Regions: Synthesis, Optical Properties and Applications. Biomolecules 2025, 15, 119. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Meng, X.; Deng, L.; Sun, Q.; Huang, X.; Lan, N.; Zhao, F. A Novel Benzothiadiazole-Based and NIR-Emissive Fluorescent Sensor for Detection of Hg2+ and Its Application in Living Cell and Zebrafish Imaging. Org. Biomol. Chem. 2020, 18, 6357–6363. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.T.; Zhang, C.; Gao, L.X.; Liu, M.M.; Yang, Y.; Shao, A.; Zhou, Y.B.; Zhu, Y.L.; Li, J.; Wang, W.L. Design, Synthesis and Evaluation of Fluorescent Properties of Benzothiazole Derivatives. ChemPhysChem 2023, 24, e202300159. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Li, Z.; Zhang, Y.; Huo, F.; Yin, C.; Liu, Y.; Li, Y.; Wang, J. A Single Fluorescent Probe for Multiple Analyte Sensing: Efficient and Selective Detection of CN−, HSO3− and Extremely Alkaline pH. J. Mater. Chem. B 2016, 4, 3703–3712. [Google Scholar] [CrossRef] [PubMed]
- Gwon, S.Y.; Rao, B.A.; Kim, H.S.; Son, Y.A.; Kim, S.H. Novel Styrylbenzothiazolium Dye-Based Sensor for Mercury, Cyanide and Hydroxide Ions. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2015, 144, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Zhai, B.; Hu, Z.; Peng, C.; Liu, B.; Li, W.; Gao, C. Rational Design of a Colorimetric and Fluorescence Turn-on Chemosensor with Benzothiazolium Moiety for Cyanide Detection in Aqueous Solution. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2020, 224, 117409. [Google Scholar] [CrossRef] [PubMed]
- Boland, M.; Pinto, S.; Gomes-da-Silva, L.C.; Pina, J.; Costa, S.P.G.; Raposo, M.M.M. Synthesis, Photophysical and Spectroscopic Characterization of Amphiphilic D-A-π-A Benzothiadiazoles. Dyes Pigm. 2025, 240, 112825. [Google Scholar] [CrossRef]
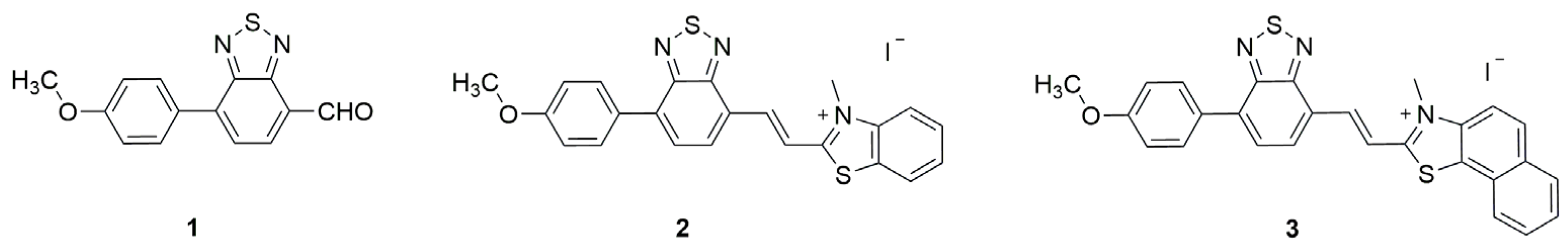




| Compound | (nm) | (nm) | (cm−1) | log ε | |
|---|---|---|---|---|---|
| 1 | 400 | 570 | 7456 | 3.99 | 0.43 |
| 2 | 465 | 657 | 6285 | 4.24 | 0.15 |
| 3 | 480 | 665 | 5796 | 4.41 | 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boland, M.L.; Costa, S.P.G.; Raposo, M.M.M. Optical Chemosensory Studies of Novel Amphiphilic D-A-π-A Benzothiadiazoles for Cyanide Detection. Eng. Proc. 2025, 118, 31. https://doi.org/10.3390/ECSA-12-26493
Boland ML, Costa SPG, Raposo MMM. Optical Chemosensory Studies of Novel Amphiphilic D-A-π-A Benzothiadiazoles for Cyanide Detection. Engineering Proceedings. 2025; 118(1):31. https://doi.org/10.3390/ECSA-12-26493
Chicago/Turabian StyleBoland, Mathilde L., Susana P. G. Costa, and M. Manuela M. Raposo. 2025. "Optical Chemosensory Studies of Novel Amphiphilic D-A-π-A Benzothiadiazoles for Cyanide Detection" Engineering Proceedings 118, no. 1: 31. https://doi.org/10.3390/ECSA-12-26493
APA StyleBoland, M. L., Costa, S. P. G., & Raposo, M. M. M. (2025). Optical Chemosensory Studies of Novel Amphiphilic D-A-π-A Benzothiadiazoles for Cyanide Detection. Engineering Proceedings, 118(1), 31. https://doi.org/10.3390/ECSA-12-26493







