Sensitive Electrochemical Detection of the Nitrite Ion Using an ISEM-3 Graphite Electrode and Comparison with Other Carbon-Containing Materials †
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
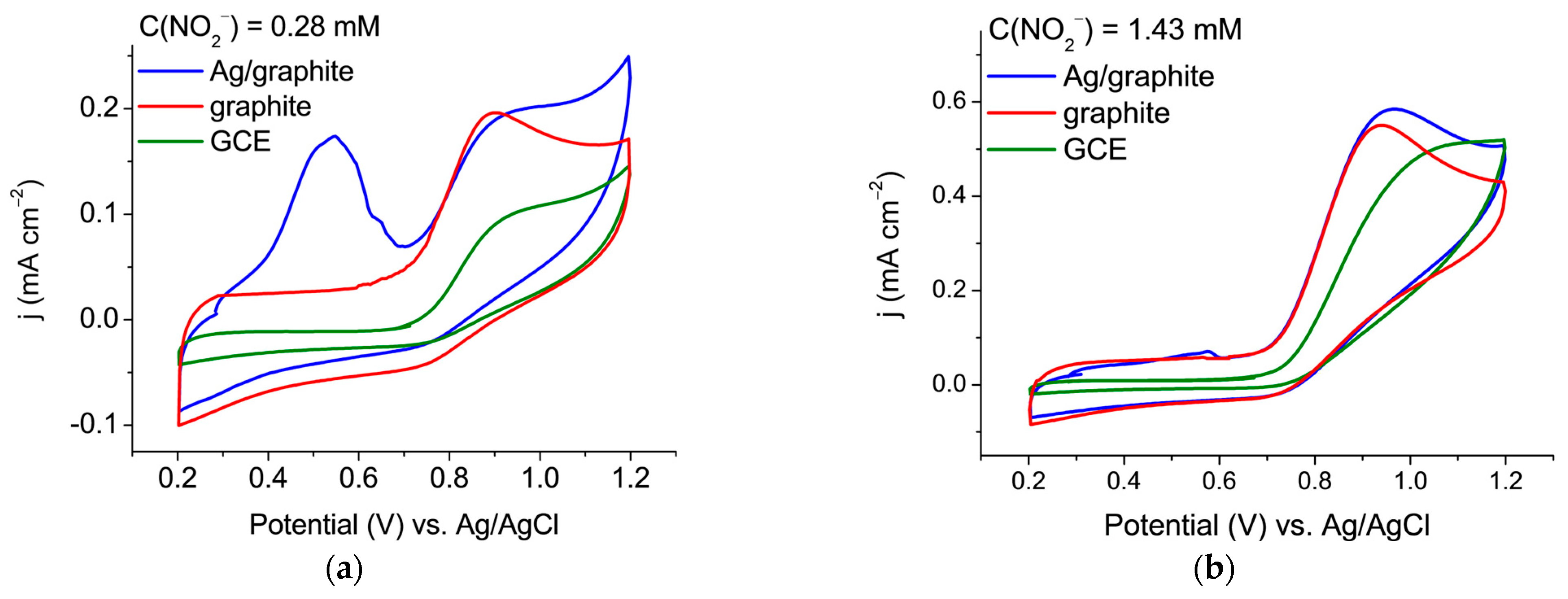
3.1. Determination of Nitrite Ions on Various Types of Carbon Electrodes
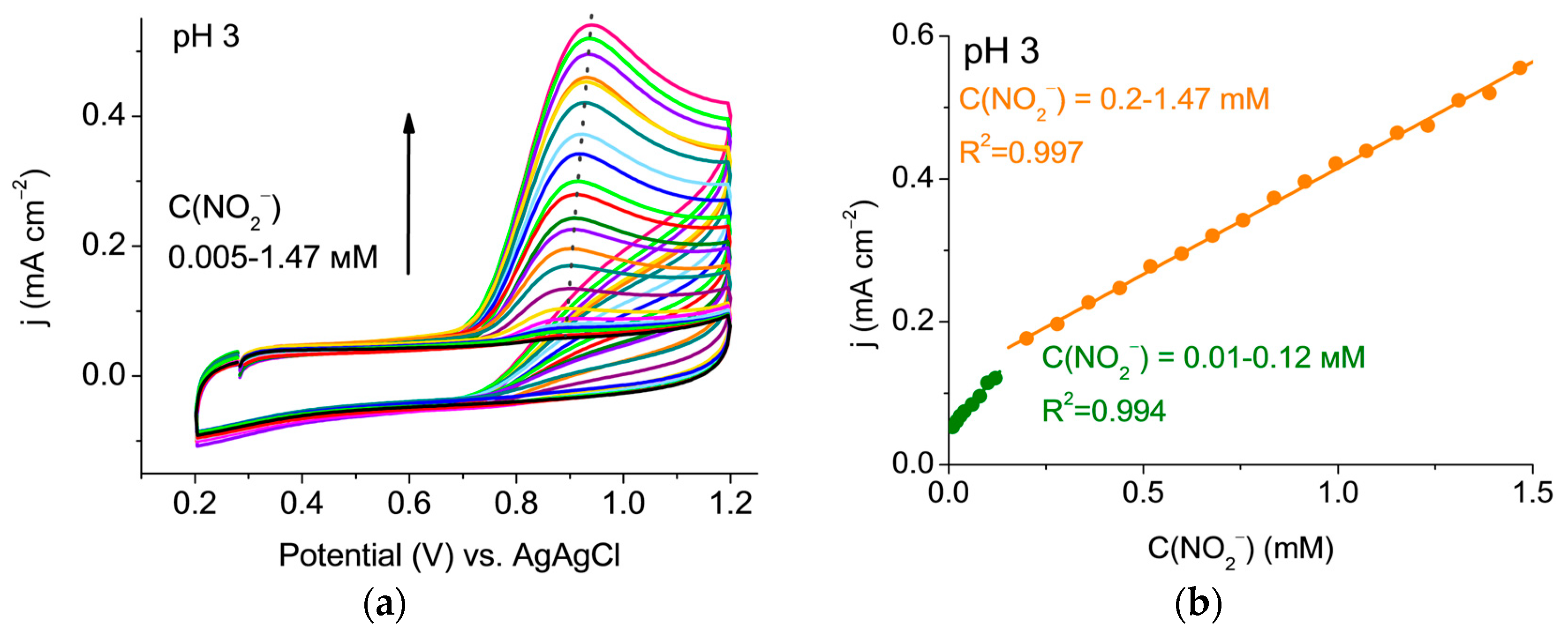
3.2. Determination of Nitrite Ions on ISEM-3 Graphite Electrode Sensor
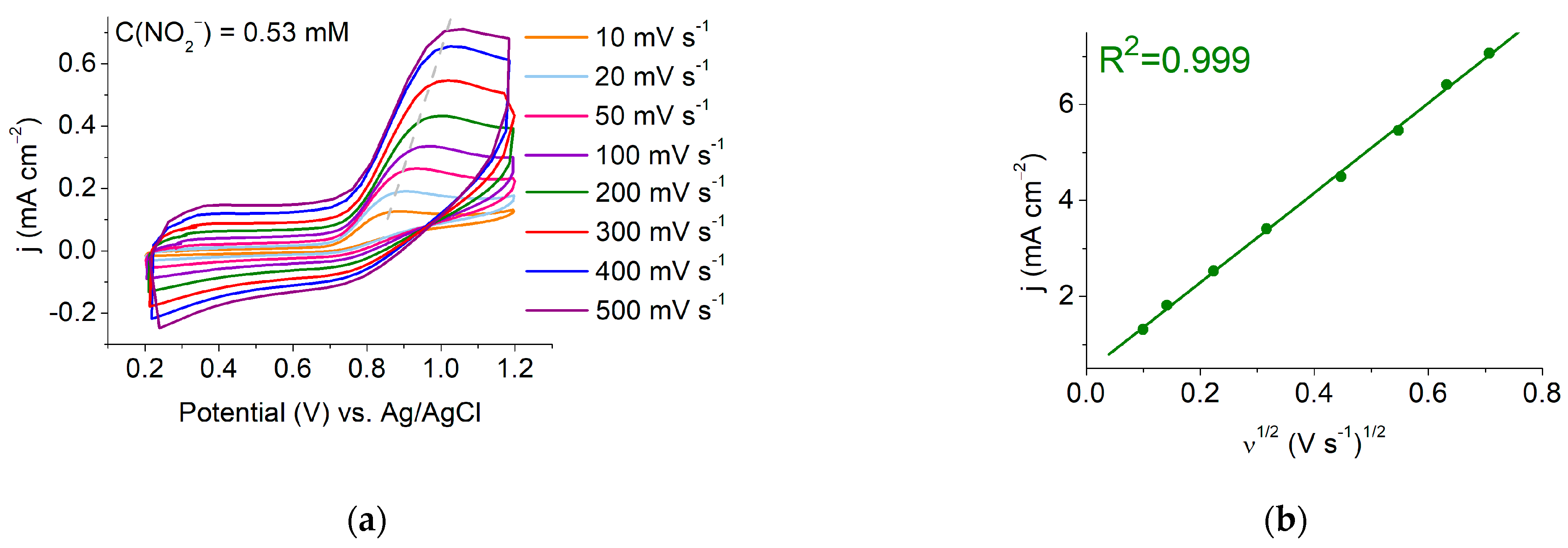
3.3. Scan Rate Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sheikh, T.; Nagendran, V.; Vasant, K.S.; Mallya, U.; Mutalik, S.; Khan, F.; Nayak, S.; Sudhakara, S.M. Acacia Auriculiformis Mediated Synthesis of Silver Nanoparticles for the Sensitive and Rapid Electrochemical Sensing of Nitrite in Water Sample. Microchem. J. 2025, 211, 113162. [Google Scholar] [CrossRef]
- Li, G.; Xia, Y.; Tian, Y.; Wu, Y.; Liu, J.; He, Q.; Chen, D. Review—Recent Developments on Graphene-Based Electrochemical Sensors toward Nitrite. J. Electrochem. Soc. 2019, 166, B881–B895. [Google Scholar] [CrossRef]
- Hefnawy, M.A.; Abdel-Gaber, R.; Gomha, S.M.; Zaki, M.E.A.; Medany, S.S. Green Synthesis of Cobalt Oxide Decorated Chitosan Substrates for Electrochemical Detection of Nitrite and Hydrogen Evolution Reactions. Electrocatalysis 2024, 15, 496–506. [Google Scholar] [CrossRef]
- Gajdár, J.; Rodrigues Gaspar, S.; Almeida, M.G. Trends in Nitrite Detection: Recent Advances in Electrochemical Sensor Technologies. TrAC Trends Anal. Chem. 2025, 183, 118105. [Google Scholar] [CrossRef]
- Mo, R.; Wang, X.; Yuan, Q.; Yan, X.; Su, T.; Feng, Y.; Lv, L.; Zhou, C.; Hong, P.; Sun, S.; et al. Electrochemical Determination of Nitrite by Au Nanoparticle/Graphene-Chitosan Modified Electrode. Sensors 2018, 18, 1986. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ping, J.; Ying, Y. Recent Developments in Carbon Nanomaterial-Enabled Electrochemical Sensors for Nitrite Detection. TrAC Trends Anal. Chem. 2019, 113, 1–12. [Google Scholar] [CrossRef]
- Tan, J.F.; Anastasi, A.; Chandra, S. Electrochemical Detection of Nitrate, Nitrite and Ammonium for on-Site Water Quality Monitoring. Curr. Opin. Electrochem. 2022, 32, 100926. [Google Scholar] [CrossRef]
- Ramesh, A.; Sahu, P.K.; Duvvuri, S.; Subrahmanyam, C. MnCo2O4 Spinel Nanorods for Highly Sensitive Electrochemical Detection of Nitrite. Inorg. Chem. 2024, 63, 9941–9952. [Google Scholar] [CrossRef] [PubMed]
- Nethravathi, P.C.; Suresh, D.; Manjula, M.V.; Devaraja, S.; Mohan, S. Ag-Cu2O Decorated Reduced Graphene Oxide Nanocomposite for Photocatalytic Water Splitting, Methylene Blue Dye Degradation, Electrochemical Nitrite Sensing, Photoluminescence and Selected Biological Applications. Biomass Convers. Biorefinery 2024, 14, 5711–5734. [Google Scholar] [CrossRef]
- Kozub, B.R.; Rees, N.V.; Compton, R.G. Electrochemical Determination of Nitrite at a Bare Glassy Carbon Electrode; Why Chemically Modify Electrodes? Sens. Actuators B 2010, 143, 539–546. [Google Scholar] [CrossRef]
- Yildiz, G.; Oztekin, N.; Orbay, A.; Senkal, F. Voltammetric Determination of Nitrite in Meat Products Using Polyvinylimidazole Modified Carbon Paste Electrode. Food Chem. 2014, 152, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Kuralay, F.; Dumangöz, M.; Tunç, S. Polymer/Carbon Nanotubes Coated Graphite Surfaces for Highly Sensitive Nitrite Detection. Talanta 2015, 144, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsova, I.; Polyakova, O.; Lebedeva, O.; Kultin, D.; Kustov, L. Sensitive Electrochemical Detection of the Nitrite Ion Using an ISEM-3 Graphite Electrode and Comparison with Other Carbon-Containing Materials. Eng. Proc. 2025, 118, 17. https://doi.org/10.3390/ECSA-12-26487
Kuznetsova I, Polyakova O, Lebedeva O, Kultin D, Kustov L. Sensitive Electrochemical Detection of the Nitrite Ion Using an ISEM-3 Graphite Electrode and Comparison with Other Carbon-Containing Materials. Engineering Proceedings. 2025; 118(1):17. https://doi.org/10.3390/ECSA-12-26487
Chicago/Turabian StyleKuznetsova, Irina, Olesya Polyakova, Olga Lebedeva, Dmitry Kultin, and Leonid Kustov. 2025. "Sensitive Electrochemical Detection of the Nitrite Ion Using an ISEM-3 Graphite Electrode and Comparison with Other Carbon-Containing Materials" Engineering Proceedings 118, no. 1: 17. https://doi.org/10.3390/ECSA-12-26487
APA StyleKuznetsova, I., Polyakova, O., Lebedeva, O., Kultin, D., & Kustov, L. (2025). Sensitive Electrochemical Detection of the Nitrite Ion Using an ISEM-3 Graphite Electrode and Comparison with Other Carbon-Containing Materials. Engineering Proceedings, 118(1), 17. https://doi.org/10.3390/ECSA-12-26487






