Integrated DFT Study of CO2 Capture and Utilization in Gingerol Extraction Using Choline Chloride–Lactic Acid Deep Eutectic Solvent †
Abstract
1. Introduction
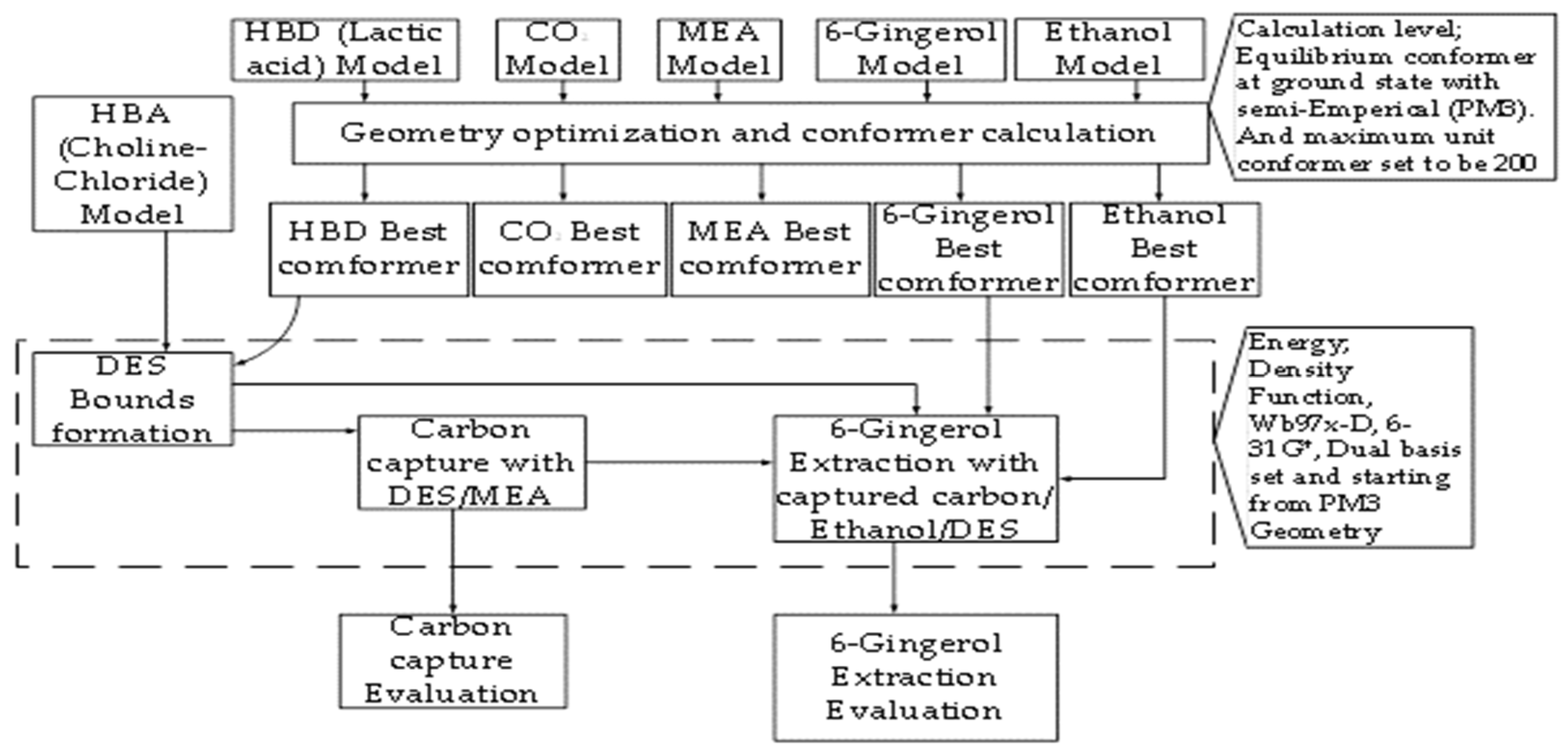
2. Computational Resources and Methodology
2.1. Computational Resources
2.2. Computational Details
2.3. Study Strategy
2.3.1. Interaction Analysis for Carbon Capture
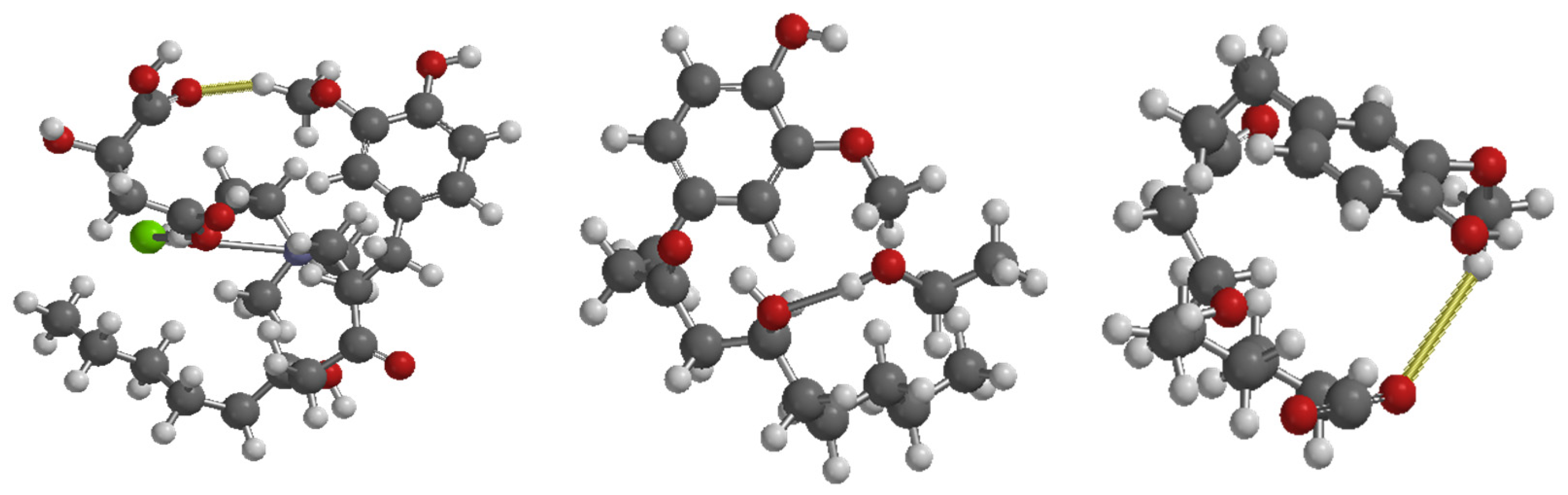
2.3.2. Interaction Analysis Between 6-Gingerol and Solvents (CO2 and DES)
3. Results and Discussions
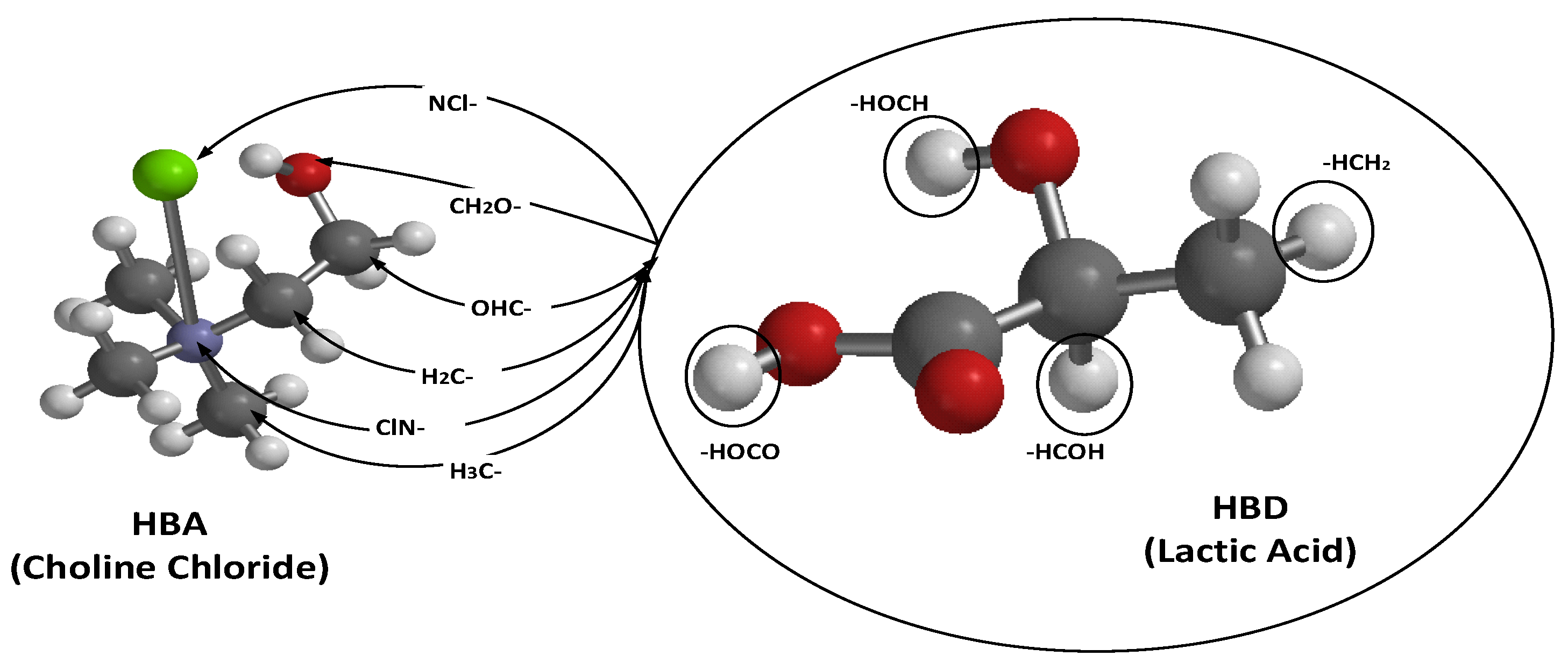
3.1. Evaluation of DES Component and Formation Mechanism
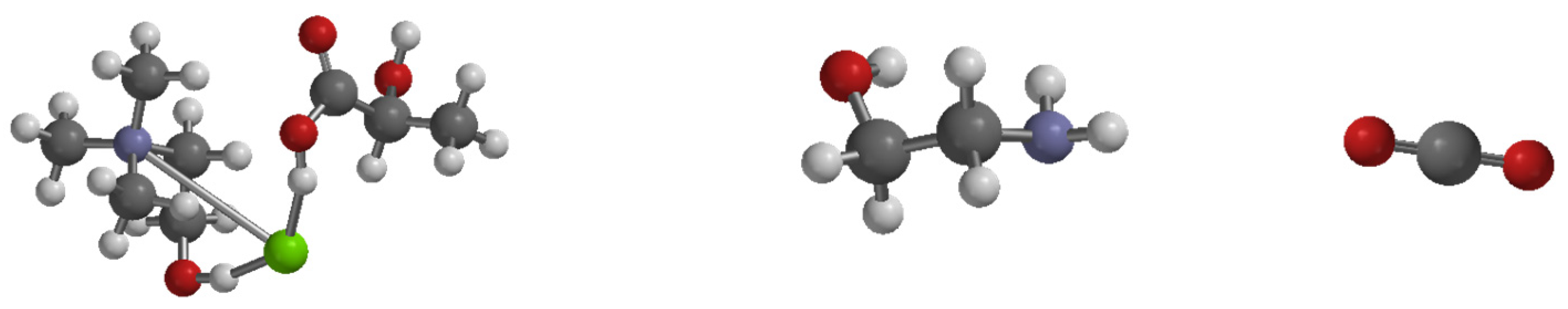
3.2. Molecular Property for Carbon Capture Using DES and MEA (Monoethanolamine)
3.3. Evaluation of Capturing Capacity of CO2
3.4. Evaluation Capacity of Gingerol Extraction
4. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rochelle, G.T. Amine Scrubbing for CO2 Capture. Science 2009, 325, 1652. [Google Scholar] [CrossRef]
- Mao, J.; Ma, Y.; Ci, Y.; Liu, J.; Li, C.; Yang, W. Anhydrous deep eutectic solvents-based biphasic absorbents for efficient CO2 capture: Unravelling the critical role of hydrogen bond-mediated electron transfer. Chem. Eng. J. 2025, 511, 161988. [Google Scholar] [CrossRef]
- Mihaila, E.G.; Aruxandei, D.C.; Doncea, S.M.; Oancea, F. Deep Eutectic Solvents for CO2 Capture in Post-Combustion Processes. Stud. UBB Chem. 2021, 2, 233–246. Available online: https://chem.ubbcluj.ro/~studiachemia/issues/chemia2021_2/20Mihaila_etal_233_246.pdf (accessed on 10 November 2025). [CrossRef]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Belwal, T.; Ezzat, S.M.; Rastrelli, L.; Bhatt, I.D.; Daglia, M.; Baldi, A.; Devkota, H.P.; Orhan, I.E.; Patra, J.K.; Das, G.; et al. A critical analysis of extraction techniques used for botanicals: Trends, priorities, industrial uses and optimization strategies. Trends Anal. Chem. J. 2018, 100, 82–102. [Google Scholar] [CrossRef]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural Deep Eutectic Solvents—Solvents for the 21st Century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Florindo, C.; Branco, L.C.; Marrucho, I.M. Development of hydrophobic deep eutectic solvents for extraction of pesticides from aqueous environments. Fluid Phase Equilib. 2017, 448, 135–142. [Google Scholar] [CrossRef]
- Gao, J.; Liu, Y.; Song, X.; Wang, S.; Ren, Y.; Xu, S. Diluent Tailored quaternary deep eutectic solvents for energy efficient carbon capture. Sep. Purif. Technol. 2025, 378, 134623. [Google Scholar] [CrossRef]
- Zheng, Q.; Liang, Z.; Li, D.; Yang, F.; Tan, H.; Wang, X. Absorption characteristics of amine-based deep eutectic solvents for CO2 capture. Sep. Purif. Technol. 2025, 378, 134654. [Google Scholar] [CrossRef]
- Oyegoke, T.; Ademola, O.; Olusanya, J.J. Preliminary Investigation on the Screening of Selected Metallic Oxides, M2O3 (M = Fe, La, and Gd) for the Capture of Carbon Monoxide Using a Computational Approach. J. Eng. Sci. Comput. 2021, 3, 1–14. [Google Scholar]
- Ademola, O.; Oyegoke, T.; John Olusanya, J. Computational Study of CO Adsorption Potential of MgO, SiO2, Al2O3, and Y2O3 Using a Semiempirical Quantum Calculation Method. Niger. J. Mater. Sci. Eng. 2021, 11, 52–57. [Google Scholar]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed]
- Stanisz, M.; Stanisz, B.J.; Cielecka-Piontek, J. A Comprehensive Review on Deep Eutectic Solvents: Their Current Status and Potential for Extracting Active Compounds from Adaptogenic Plants. Molecules 2024, 29, 4767. [Google Scholar] [CrossRef]
- Schröder, H.; Hühnert, J.; Schwabe, T. Evaluation of DFT-D3 dispersion corrections for various structural benchmark sets. J. Chem. Phys. 2017, 146, 044115. [Google Scholar] [CrossRef] [PubMed]
- Uzochukwu, M.I.; Oyegoke, T.; Momoh, R.O.; Isa, M.T.; Shuwa, S.M.; Jibril, B.Y. Computational insights into deep eutectic solvent design: Modeling interactions and thermodynamic feasibility using choline chloride & glycerol. Chem. Eng. J. Adv. 2023, 16, 100564. [Google Scholar] [CrossRef]
- Oyegoke, T.; Aliyu, A.; Uzochuwu, M.I.; Hassan, Y. Enhancing hydrogen sulphide removal efficiency: A DFT study on selected functionalized graphene-based materials. Carbon Trends 2024, 15, 100362. [Google Scholar] [CrossRef]
- Oyegoke, T.; Dabai, F.; Adamu, U.; Baba, Y.J. Quantum mechanics calculation of molybdenum and tungsten influence on the CrM-oxide catalyst acidity. Hittite J. Sci. Eng. 2020, 7, 297–311. [Google Scholar] [CrossRef]
- Bendjeddou, A.; Abbaz, T.; Gouasmia, A.; Villemin, D. Molecular Structure, HOMO-LUMO, MEP and Fukui Function Analysis of Some TTF-donor Substituted Molecules Using DFT (B3LYP) Calculations. Int. Res. J. Pure Appl. Chem. 2016, 12, 1–9. [Google Scholar] [CrossRef]
- Santra, M.; Kunzru, D.; Rabari, D. Understanding the interactions between CO2 and selected choline-based deep eutectic solvents using density functional theory. Fluid Phase Equilib. J. 2024, 580, 114038. [Google Scholar] [CrossRef]
- Ishaq, M.; Amjad, M.; Ahmad, F.; Muhammad, Z.; Arshad, I.; Roil, M.; Bilad, M.R.; Ayub, K.; Khan, A.L. Theoretical and experimental investigation of CO2 capture through choline chloride based supported deep eutectic liquid membranes. J. Mol. Liq. 2021, 335, 116234. [Google Scholar] [CrossRef]
- Olusola Ibraheem, A.; Toyese, O. DFT Fukui Descriptor-Based Prediction of Arsenic Adsorption on Graphene. Eur. J. Mater. Sci. Eng. 2025, 10, 181–194. [Google Scholar] [CrossRef]
- Ivanovic, M.; Islamčević Razboršek, M.; Kolar, M. Innovative Extraction Techniques Using Deep Eutectic Solvents and Analytical Methods for the Isolation and Characterization of Natural Bioactive Compounds from Plant Material. Plants 2000, 9, 1428. [Google Scholar] [CrossRef]
- Tzani, A.; Kalafateli, S.; Tatsis, G.; Rozaria, A.; Pontillo, N.; Detsi, A. Natural Deep Eutectic Solvents (NaDESs) as Alternative Green Extraction Media for Ginger (Zingiber officinale Roscoe). Sustain. Chem. 2021, 2, 576–598. [Google Scholar] [CrossRef]
- Rezaee, P.; Asl, S.A.; Javadi, M.H.; Rezaee, S.; Morad, R.; Akbari, M.; Arab, S.S.; Maaza, M. DFT study on CO2 capture using boron, nitrogen, and phosphorus-doped C20 in the presence of an electric field. Sci Rep 2024, 14, 12388. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, J.O.L.; del Castillo Vázquez, R.M.; Ramirez-de-Arellano, J.M. CO2 Absorption on Cu-Doped Graphene, a DFT Study. Crystals 2025, 15, 460. [Google Scholar] [CrossRef]
- Gehringer, D.; Dengg, T.; Popov, M.N.; Holec, D. Interactions between a H2 Molecule and Carbon Nanostructures: A DFT Study. C 2020, 6, 16. [Google Scholar] [CrossRef]

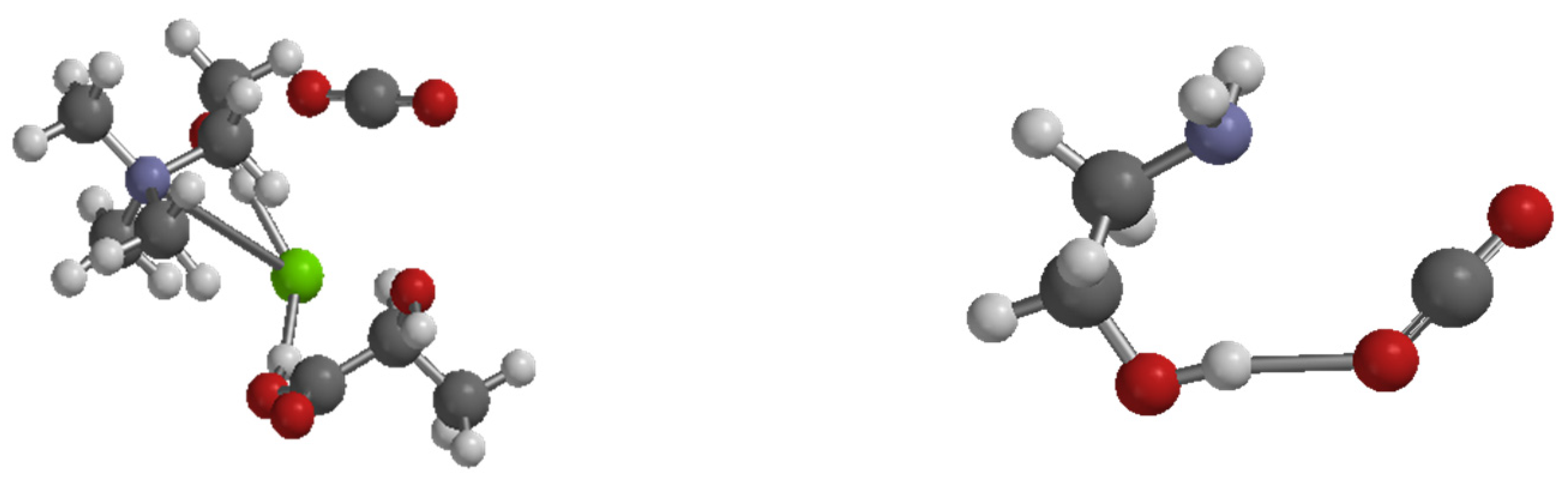
| DES Component | Molecular Formula | HOMO, H (eV) | LUMO, L (eV) | EGap (eV) abs of (H-L) | IEG-CHL | DES’s FE (eV) | |
|---|---|---|---|---|---|---|---|
| abs of (HCHL-L) | abs of (H-LCHL) | ||||||
| CHL | C5H14ClNO | −7.83 | 3.04 | 10.87 | 10.87 | 10.87 | −1.92 |
| LAC | C3H6O3 | −10.18 | 0.99 | 11.17 | 8.82 | 13.22 | |
| Species | HOMO (eV) | LUMO (eV) | 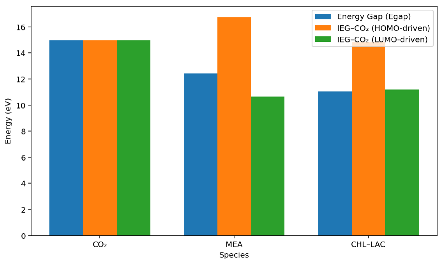 |
| CO2 | −13.13 | 1.85 | |
| MEA | −8.80 | 3.62 | |
| CHL-LAC | −9.34 | 1.71 |
| Solvent | Molecular Formula | Binding Energy |
|---|---|---|
| MEA | C3H7NO3 | −0.23 |
| CHL-LAC | C8H20ClNO4 | −0.86 |
| Species | HOMO (eV) | LUMO (eV) | 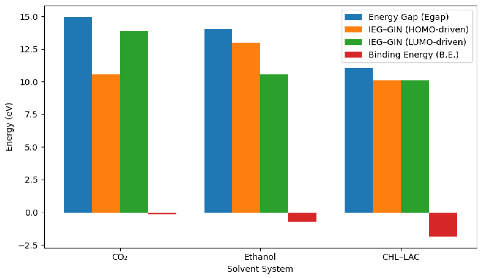 |
| CO2 | −13.13 | 1.85 | |
| GIN | −8.70 | 0.77 | |
| Ethanol | −9.77 | 4.28 | |
| CHL-LAC | −9.34 | 1.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2026 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olatunde, A.; Oyegoke, T. Integrated DFT Study of CO2 Capture and Utilization in Gingerol Extraction Using Choline Chloride–Lactic Acid Deep Eutectic Solvent. Eng. Proc. 2025, 117, 30. https://doi.org/10.3390/engproc2025117030
Olatunde A, Oyegoke T. Integrated DFT Study of CO2 Capture and Utilization in Gingerol Extraction Using Choline Chloride–Lactic Acid Deep Eutectic Solvent. Engineering Proceedings. 2025; 117(1):30. https://doi.org/10.3390/engproc2025117030
Chicago/Turabian StyleOlatunde, Abdulsobur, and Toyese Oyegoke. 2025. "Integrated DFT Study of CO2 Capture and Utilization in Gingerol Extraction Using Choline Chloride–Lactic Acid Deep Eutectic Solvent" Engineering Proceedings 117, no. 1: 30. https://doi.org/10.3390/engproc2025117030
APA StyleOlatunde, A., & Oyegoke, T. (2025). Integrated DFT Study of CO2 Capture and Utilization in Gingerol Extraction Using Choline Chloride–Lactic Acid Deep Eutectic Solvent. Engineering Proceedings, 117(1), 30. https://doi.org/10.3390/engproc2025117030








