Enzyme-Assisted Extraction of Bioactive Compounds from Origanum dictamnus L. †
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemical and Reagents
2.3. Enzyme Preparation
2.4. Enzyme-Assisted Extraction
2.5. Conventional Extraction
2.6. Total Phenolic Content
2.7. Total Flavonoid Content
2.8. Taguchi Method
3. Results and Discussion
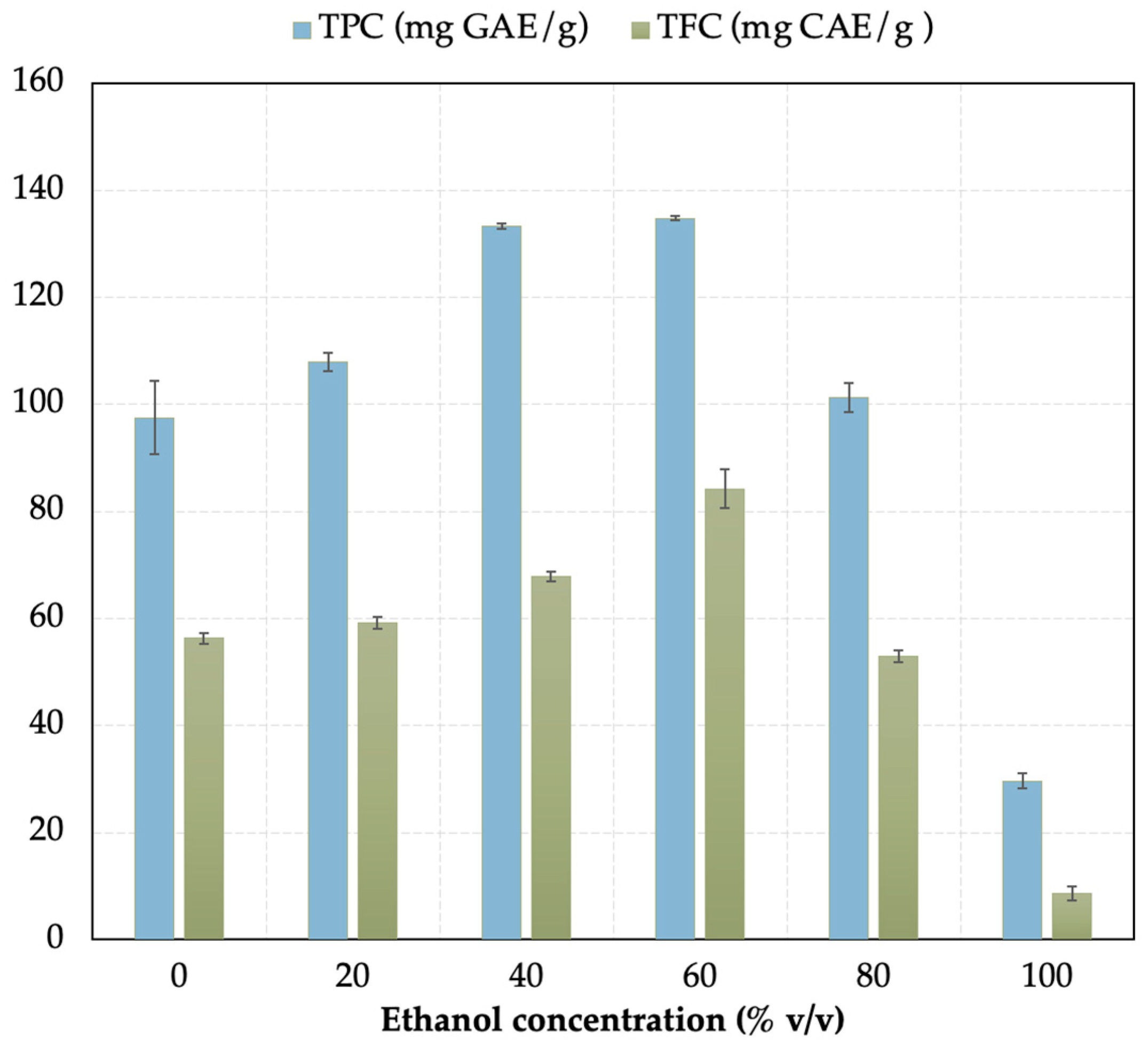
3.1. Conventional Extraction
3.2. Taguchi Method
3.2.1. Signal-to-Noise Ratios
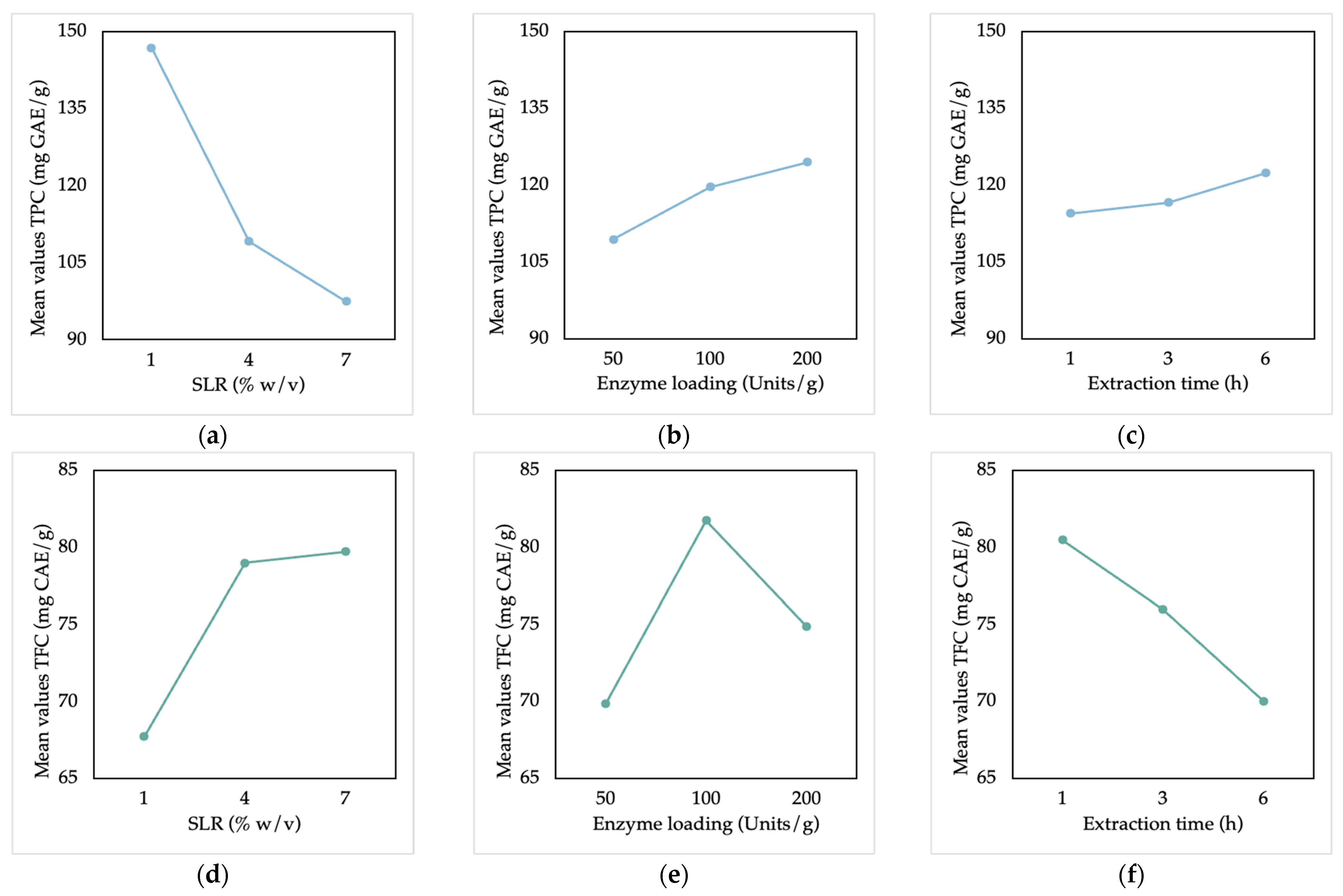
3.2.2. Optimization and Confirmation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EAE | Enzyme-assisted extraction |
| TPC | Total phenolic content |
| TFC | Total flavonoid content |
| GAE | Gallic acid equivalents |
| CAE | Catechin equivalents |
| S/N | Signal-to-noise ratio |
| SLR | Solid-to-liquid ratio |
References
- Liolios, C.C.; Graikou, K.; Skaltsa, E.; Chinou, I. Dittany of Crete: A Botanical and Ethnopharmacological Review. J. Ethnopharmacol. 2010, 131, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Solomou, A.D.; Fountouli, A.; Molla, A.; Petrakis, M.; Manolikaki, I.; Skoufogianni, E. Ecology, Cultivation, and Utilization of the Dittany of Crete (Origanum dictamnus L.) from Ancient Times to the Present: A Short Review. Agronomy 2024, 14, 1066. [Google Scholar] [CrossRef]
- Letsiou, S.; Trapali, M.; Vougiouklaki, D.; Tsakni, A.; Antonopoulos, D.; Houhoula, D. Antioxidant Profile of Origanum dictamnus L. Exhibits Antiaging Properties against UVA Irradiation. Cosmetics 2023, 10, 124. [Google Scholar] [CrossRef]
- da Silva, R.F.; Carneiro, C.N.; Cheila, C.B.; Gomez, F.J.V.; Espino, M.; Boiteux, J.; de los, Á.; Fernández, M.; Silva, M.F.; de S. Dias, F. Sustainable Extraction Bioactive Compounds Procedures in Medicinal Plants Based on the Principles of Green Analytical Chemistry: A Review. Microchem. J. 2022, 175, 107184. [Google Scholar] [CrossRef]
- Nadar, S.S.; Rao, P.; Rathod, V.K. Enzyme Assisted Extraction of Biomolecules as an Approach to Novel Extraction Technology: A Review. Food Res. Int. 2018, 108, 309–330. [Google Scholar] [CrossRef] [PubMed]
- Lemoni, Z.; Kalantzi, S.; Lymperopoulou, T.; Tzani, A.; Stavropoulos, G.; Detsi, A.; Mamma, D. Optimization of Bioactive Compounds Extraction from Rosa canina L. Pseudofruit through the Action of Two Hydrolytic Enzyme Preparations. J. Chem. Technol. Biotechnol. 2025, 1–14. [Google Scholar] [CrossRef]
- Gligor, O.; Mocan, A.; Moldovan, C.; Locatelli, M.; Crișan, G.; Ferreira, I.C.F.R. Enzyme-Assisted Extractions of Polyphenols—A Comprehensive Review. Trends Food Sci. Technol. 2019, 88, 302–315. [Google Scholar] [CrossRef]
- Lemoni, Z.; Kalantzi, S.; Lymperopoulou, T.; Tzani, A.; Stavropoulos, G.; Detsi, A.; Mamma, D. Kinetic Modeling and Biological Activities of Rosa canina L. Pseudo-Fruit Extracts Obtained via Enzyme-Assisted Extraction. Antioxidants 2025, 14, 558. [Google Scholar] [CrossRef] [PubMed]
- Meena, K.; Kumar, A.; Pandya, S.N.; Pandya, S.N. Optimization of Friction Stir Processing Parameters for 60/40 Brass Using Taguchi Method. Mater. Today Proc. 2017, 4, 1978–1987. [Google Scholar] [CrossRef]
- Idris, F.N.; Nadzir, M.M.; Abd Shukor, S.R. Optimization of Solvent-Free Microwave Extraction of Centella Asiatica Using Taguchi Method. J. Environ. Chem. Eng. 2020, 8, 103766. [Google Scholar] [CrossRef]
- Biesaga, M. Influence of Extraction Methods on Stability of Flavonoids. J. Chromatogr. A 2011, 1218, 2505–2512. [Google Scholar] [CrossRef] [PubMed]

| A/A | Solid to Liquid Ratio (% w/v) | Enzyme Loading (Units/g) | Extraction Time (h) | TPC (mg GAE/g) | TFC (mg CAE/g) |
|---|---|---|---|---|---|
| 1 | 1 | 50 | 1 | 131.6 ± 2.8 | 62.5 ± 6.1 |
| 2 | 1 | 100 | 3 | 144.2 ± 0.6 | 77.5 ± 6.6 |
| 3 | 1 | 200 | 6 | 164.8 ± 5.2 | 63.2 ± 5.6 |
| 4 | 4 | 50 | 3 | 106.2 ± 2.8 | 75.4 ± 0.8 |
| 5 | 4 | 100 | 6 | 112.0 ± 2.4 | 75.2 ± 2.9 |
| 6 | 4 | 200 | 1 | 109.3 ± 3.6 | 86.4 ± 5.1 |
| 7 | 7 | 50 | 6 | 90.3 ± 5.2 | 71.7 ± 2.4 |
| 8 | 7 | 100 | 1 | 102.6 ± 7.4 | 92.5 ± 5.7 |
| 9 | 7 | 200 | 3 | 99.4 ± 0.7 | 75.0 ± 3.1 |
| Run | S/N TPC | S/N TFC |
|---|---|---|
| 1 | 42.38 | 35.92 |
| 2 | 43.18 | 37.78 |
| 3 | 44.34 | 36.01 |
| 4 | 40.52 | 37.55 |
| 5 | 41.00 | 37.53 |
| 6 | 40.77 | 38.73 |
| 7 | 39.11 | 37.11 |
| 8 | 40.22 | 39.32 |
| 9 | 39.95 | 37.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemoni, Z.; Leka, R.K.; Lymperopoulou, T.; Mamma, D. Enzyme-Assisted Extraction of Bioactive Compounds from Origanum dictamnus L. Eng. Proc. 2025, 117, 2. https://doi.org/10.3390/engproc2025117002
Lemoni Z, Leka RK, Lymperopoulou T, Mamma D. Enzyme-Assisted Extraction of Bioactive Compounds from Origanum dictamnus L. Engineering Proceedings. 2025; 117(1):2. https://doi.org/10.3390/engproc2025117002
Chicago/Turabian StyleLemoni, Zafeiria, Roza Konstantina Leka, Theopisti Lymperopoulou, and Diomi Mamma. 2025. "Enzyme-Assisted Extraction of Bioactive Compounds from Origanum dictamnus L." Engineering Proceedings 117, no. 1: 2. https://doi.org/10.3390/engproc2025117002
APA StyleLemoni, Z., Leka, R. K., Lymperopoulou, T., & Mamma, D. (2025). Enzyme-Assisted Extraction of Bioactive Compounds from Origanum dictamnus L. Engineering Proceedings, 117(1), 2. https://doi.org/10.3390/engproc2025117002







