Characterization of Pressureless Sintering of ZTA Ceramic †
Abstract
1. Introduction
- To lower the sintering temperature of ZTA by adding a suitable additive (MgO).
- To observe the change in the properties of ZTA using an optimum ratio of zirconia (ZrO2).
- To observe the pressureless sintering of ZTA.
Research Strategy
- The preparation of green powders by mixing an optimum ratio of zirconia in alumina powder.
- Ball milling of the mixed powder to ensure a homogenous mixture and reduce the particle size.
- The compaction of the powder to obtain cylindrical pellets and measure the green state density.
- Mixing the powder with the 3d-printed resin with different ratios.
- Sintering at different temperatures, i.e., 1500 °C and 1600 °C.
- Measuring the properties of sintered ZTA samples (density and hardness).
2. Materials and Methods
2.1. Materials Used
- Pure alumina powder (99% Al2O3).
- Zirconia powder (ZrO2).
- Magnesium Oxide powder (MgO).
- Stearic Acid (C18H36O2) as a binder.
- Pure ethanol (99% C2H5OH).
- 3d—printing UV-sensitive resin (UV wavelength 405 nm).
2.2. Powder Development
2.2.1. Ceramic Composition
2.2.2. Ball Milling
2.3. Green State Pressing
2.4. Sintering
2.5. Furnace
Box Chamber Furnace
2.6. Density Measurement
2.7. Hardness Measurement
3. Results
4. Conclusions
- The powder size and homogenous mixing play an important role in determining the properties of the final sintered product. Hence, ball milling is important to ensure small particle sizes and the homogenous mixing of the green powder.
- Different concentrations of zirconia in alumina, i.e., 5–19%, produced different results, so the right concentration of zirconia should be used.
- Increasing the sintering temperature produced better relative densities.
- Viscosity plays an important role in determining the 3d printing capability of the powder–resin mixture.
- The right balance between the viscosity and density should be considered for the powder–resin mixture.
- At 1500 °C a relative density up to 70% was obtained.
- The hardness range (i.e., 14–17 GPa) is due to the difference in the microstructure between the samples at the given temperature (i.e., 1500 °C and 1600 °C).
- The addition of MgO improved the sinterablity and densification of the samples.
- When 1.5 g of ZTA powder was mixed with 1 mL of the UV-curable resin (405 nm), the resulting mixture had an approximate volume of 2.5 cm3. The sample cured in 6 min under UV exposure, whereas the pure resin’s curing time was 4 min.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marti, A. Inert bioceramics (AI2O3, ZrO2) for medical application. Inj. Int. J. Care Inj. 2000, 31, D33–D36. [Google Scholar]
- Mutsuddy, B.C. Influence of Powder Characteristics on the Rheology of Ceramic Injection Molding Mixtures. Proc. Brit. Ceram. Soc. 1983, 33, 117–137. [Google Scholar]
- Thomas, P.; Barnstorf, S.; Summer, B.; Willmann, G.; Przybilla, B. Immuno-allergological properties of aluminium oxide (Al2O3) ceramics and nickel sulfate in humans. Biomaterials 2003, 24, 959–966. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, J. What future for zirconia as biomaterials? Biomaterials 2006, 27, 535–543. [Google Scholar] [CrossRef]
- Piotter, V.; Beck, M.B.; Ritzhaupt-Kleissl, H.J.; Ruh, A.; Hausselt, J. Recent developments in micro ceramic injection molding. Int. J. Mater. Res. 2008, 99, 1157–1162. [Google Scholar] [CrossRef]
- Rogner, J.; Okolo, B.; Kurzenhäuser, S.; Müller, M.; Bauer, W.; Ritzhaupt-Kleissl, H.J.; Kerscher, E.; Schulze, V. Relationships between process, microstructure and properties of molded zirconia micro specimens. Microsyst. Technol. 2008, 14, 1831–1837. [Google Scholar] [CrossRef]
- Kasanicka, B.; Müller, M.; Auhorn, M.; Schulze, V.; Bauer, W.; Beck, T.; Ritzhaupt-Kleissl, H.J.; Löhe, D. Correlations between production process, states and mechanical properties of microspecimens made of zirconia. Microsyst. Technol. 2006, 12, 1133–1141. [Google Scholar] [CrossRef]
- Bae, I.J.; Baik, S. Abnormal Grain Growth of Alumina. J. Am. Ceram. Soc. 1997, 80, 1149–1156. [Google Scholar] [CrossRef]
- Yu, P.C.; Li, Q.F.; Fuh, J.Y.H.; Li, T.; Ho, P.W. Micro injection molding of micro gear using nano-sized zirconia powder. Microsyst. Technol. 2009, 15, 401–406. [Google Scholar] [CrossRef]
- Claussen, N.; Petzow, G. Whisker-Reinforced Zirconia-Toughened Ceramics. In Tailoring Multiphase and Composite Ceramics, 1st ed.; Tressler, R.E., Ed.; Springer: New York, NY, USA, 1986; Volume 20. [Google Scholar]
- Kriven, W.M. The Transformation Mechanism of Spherical Zirconia Particles in Alumina. In Advances in Ceramics, Vol. 12, Science and Technology of Zirconia II; American Ceramic Society, Inc.: Columbus, OH, USA, 1984; Volume 12, pp. 64–77. [Google Scholar]
- Tiegs, T.; Becher, P. Whisker-Reinforced Ceramic Composites. In Tailoring Multiphase and Composite Ceramics; Tressler, R.E., Ed.; Plenum Press: New York, NY, USA, 1986; Volume 20. [Google Scholar]
- Wang, C.J.; Huang, C.Y.; Wu, Y.C. Two-step sintering of fine alumina–zirconia ceramics. Ceram. Int. 2009, 35, 1467–1472. [Google Scholar] [CrossRef]
- Walker, L.S.; Marotto, V.R.; Rafiee, M.A.; Koratkar, N.; Corral, E.L. Toughening in Graphene Ceramic Composites. ACS Nano 2011, 5, 3182–3190. [Google Scholar] [CrossRef]
- Simpson, C.J.; Aust, K.T.; Winegard, W.C. 4 Stages of Grain Growth. Metall. Trans. 1971, 2, 987–991. [Google Scholar] [CrossRef]
- Garvie, R.C.; Hannink, R.H.J.; Pascoe, R.T. Ceramic Steel? Nature 1975, 258, 703–704. [Google Scholar] [CrossRef]
- McMeeking, R.M.; Evans, A.G. Mechanics of Transformation-Toughening in Brittle Materials. J. Am. Ceram. Soc. 1982, 65, 242–246. [Google Scholar] [CrossRef]
- Faber, K.T.; Evans, A.G. Crack Deflection Processes—I. Theory. Acta Metall. 1983, 31, 565–576. [Google Scholar] [CrossRef]
- Das, K.; Banerjee, G. Mechanical Properties and Microstructures of Reaction Sintered Mullite- Zirconia Composites in the Presence of an Additive—Dysprosia. J. Eur. Ceram. Soc. 2000, 20, 153–157. [Google Scholar] [CrossRef]
- Suryanarayana, C. Nanocrystalline Materials. Int. Mat. Rev. 1995, 40, 41–64. [Google Scholar] [CrossRef]
- He, Z.; Ma, J. Grain Growth Rate Constant of Hot-Pressed Alumina Ceramics. Mater. Lett. 2000, 44, 14–18. [Google Scholar] [CrossRef]
- Porat, R.; Berger, S.; Rosen, A. Dilatometric Study of the Sintering Mechanism of Nanocrystalline Cemented Carbides. Nano Mater. 1996, 7, 429–436. [Google Scholar] [CrossRef]
- Gao, L.; Hong, J.S.; Miyamoto, H.; Torre, D.D.L. Bending Strength and Microstructure of Al2O3 Ceramics Densified by Spark Plasma Sintering. J. Eur. Ceram. Soc. 2000, 20, 2149–2152. [Google Scholar] [CrossRef]
- Chakravarty, D.; Bysakh, S.; Muraleedharan, K.; Rao, T.N.; Sundaresan, R. Spark Plasma Sintering of Magnesia-Doped Alumina with High Hardness and Fracture Toughness. J. Am. Ceram. Soc. 2008, 91, 203–208. [Google Scholar] [CrossRef]
- Zhou, Y.; Hirao, K.; Yamauchi, Y.; Kanzaki, S. Densification and Grain Growth in Pulse Electric Current Sintering of Alumina. J. Eur. Ceram. Soc. 2004, 24, 3465–3470. [Google Scholar] [CrossRef]

| Substance | % Composition |
|---|---|
| Polyurethane acrylate | 55 |
| Acrylate Monomer | 40 |
| Photoinitiator | 5 |
| Property Name | Value |
|---|---|
| Density (g/cm3) | 0.8–0.9 |
| Viscosity (mPa.s) | 150–250 |
| Boiling Point (°C) | 238 |
| Odor | Odorless |
| Milling Media | Milling Ratio | Milling Time | Drying |
|---|---|---|---|
| WC Balls/Ethanol | 1:3 | 4 h@100 rpm | 80 °C for 5.5 h |
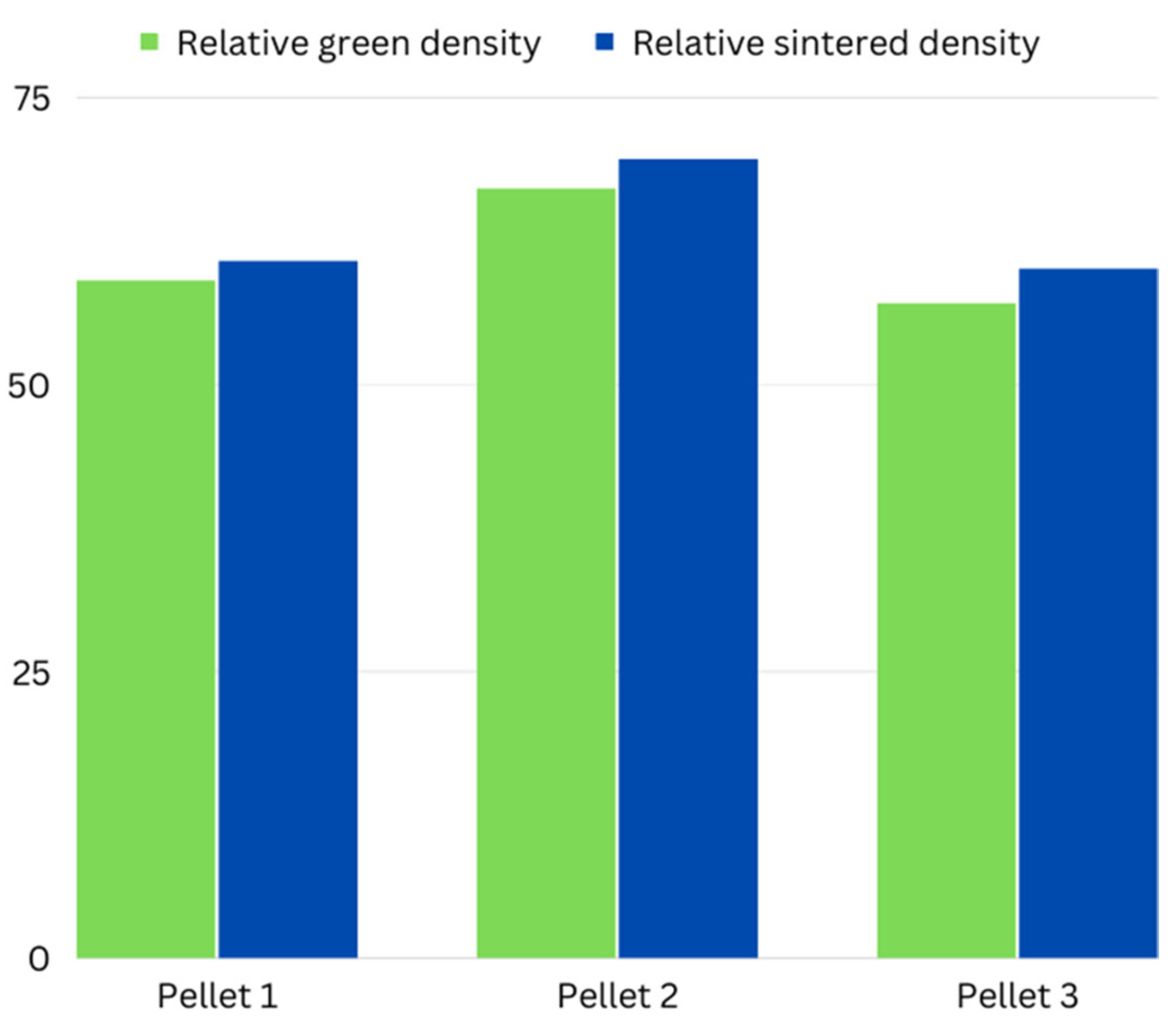
| Parameters | Pellet 1 | Pellet 2 | Pellet 3 |
|---|---|---|---|
| Green Product | W = 5 g | W = 6 g | W = 5 g |
| H = 0.971 cm | H = 1.029 cm | H = 1.003 cm | |
| D = 1.612 cm | D = 1.612 cm | D = 1.618 cm | |
| Sintered Product | W = 5 g | W = 6 g | W = 5 g |
| H = 0.967 cm | H = 1.012 cm | H = 0.973 cm | |
| D = 1.596 cm | D = 1.596 cm | D = 1.597 cm | |
| Sintered Density | ρ = 2.586 g/cm3 | ρ = 2.965 g/cm3 | ρ = 2.567 g/cm3 |
| Sintered Density (%) | ρ = 60.70% | ρ = 69.59% | ρ = 60% |
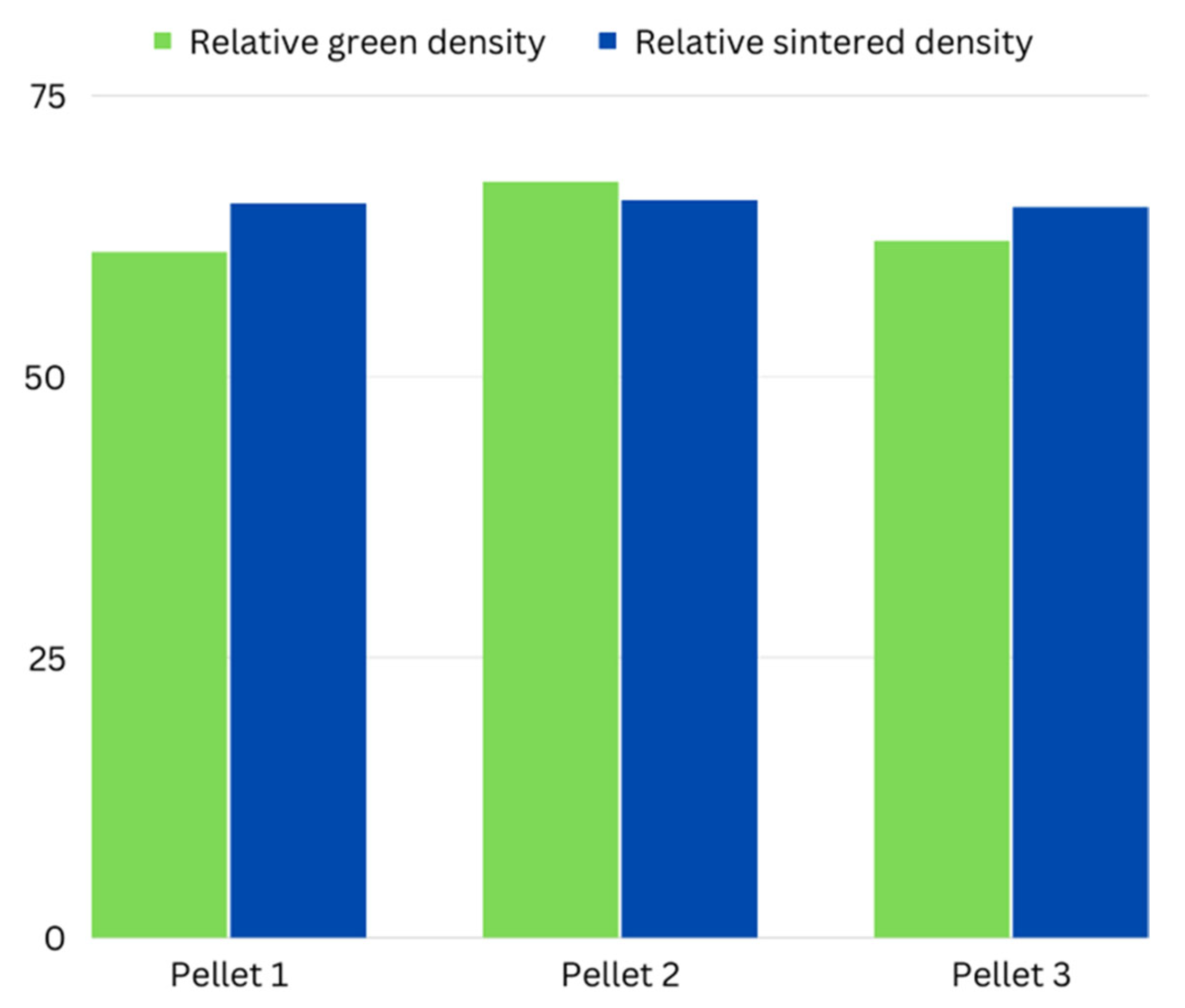
| Parameters | Pellet 1 | Pellet 2 | Pellet 3 |
|---|---|---|---|
| Green Product | W = 4.88 g | W = 4.91 g | W = 4.90 g |
| H = 0.923 cm | H = 0.84 cm | H = 0.908 cm | |
| D = 1.609 cm | D = 1.611 cm | D = 1.611 cm | |
| Sintered Product | W = 4.81 g | W = 4.84 g | W = 4.81 g |
| H = 0.904 cm | H = 0.893 cm | H = 0.907 cm | |
| D = 1.560 cm | D = 1.571 cm | D = 1.564 cm | |
| Sintered Density | ρ = 2.785 g/cm3 | ρ = 2.798 g/cm3 | ρ = 2.762 g/cm3 |
| Sintered Density (%) | ρ = 65.30% | ρ = 65.6% | ρ = 65% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rafay, A.; Shah, O.u.R.; Ahmad, N. Characterization of Pressureless Sintering of ZTA Ceramic. Eng. Proc. 2025, 111, 20. https://doi.org/10.3390/engproc2025111020
Rafay A, Shah OuR, Ahmad N. Characterization of Pressureless Sintering of ZTA Ceramic. Engineering Proceedings. 2025; 111(1):20. https://doi.org/10.3390/engproc2025111020
Chicago/Turabian StyleRafay, Abdul, Owais ur Rehman Shah, and Naseem Ahmad. 2025. "Characterization of Pressureless Sintering of ZTA Ceramic" Engineering Proceedings 111, no. 1: 20. https://doi.org/10.3390/engproc2025111020
APA StyleRafay, A., Shah, O. u. R., & Ahmad, N. (2025). Characterization of Pressureless Sintering of ZTA Ceramic. Engineering Proceedings, 111(1), 20. https://doi.org/10.3390/engproc2025111020






