Comparison of Modifications of Cellulose for the Extraction and Elution of DNA †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Salmon Sperm Sonication
2.2.2. Paper Treatments
2.2.3. Paper-DNA Interaction Analyses
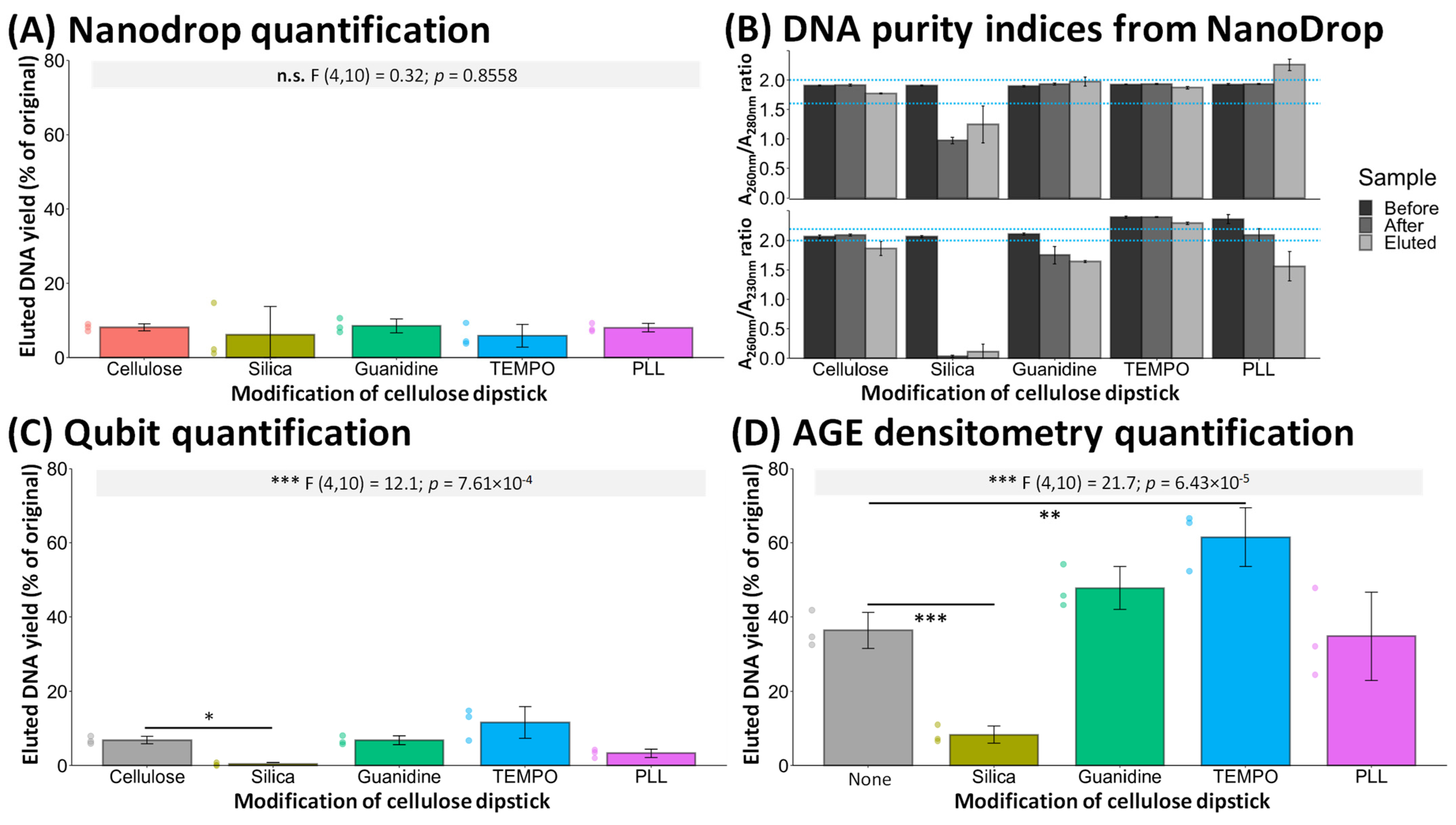
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huan, T.; Joehanes, R.; Song, C.; Peng, F.; Guo, Y.; Mendelson, M.; Yao, C.; Liu, C.; Ma, J.; Richard, M.; et al. Genome-wide identification of DNA methylation QTLs in whole blood highlights pathways for cardiovascular disease. Nat. Commun. 2019, 10, 4267. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Han, D.Y.; Fraser, A.G.; Huebner, C.; Lam, W.J.; Morgan, A.R.; Duan, H.; Karunasinghe, N. Genetic factors in chronic inflammation: Single nucleotide polymorphisms in the STAT-JAK pathway, susceptibility to DNA damage and Crohn’s disease in a New Zealand population. Mutat. Res. Mol. Mech. Mutagen. 2010, 690, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Tettamanti, L.; Gaudio, R.M.; Iapichino, A.; Mucchi, D.; Tagliabue, A. Genetic susceptibility and periodontal disease: A retrospective study on a large Italian sample. Oral Implant. 2017, 10, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Hwang, G.T. DNA-Encoded Library Screening as Core Platform Technology in Drug Discovery: Its Synthetic Method Development and Applications in DEL Synthesis. J. Med. Chem. 2020, 63, 6578–6599. [Google Scholar] [CrossRef]
- Luna, R.A.; Fasciano, L.R.; Jones, S.C.; Boyanton, B.L.; Ton, T.T.; Versalovic, J. DNA Pyrosequencing-Based Bacterial Pathogen Identification in a Pediatric Hospital Setting. J. Clin. Microbiol. 2007, 45, 2985–2992. [Google Scholar] [CrossRef]
- Chong, K.W.Y.; Thong, Z.; Syn, C.K.-C. Recent trends and developments in forensic DNA extraction. Wiley Interdiscip. Rev. Forensic Sci. 2021, 3, e1395. [Google Scholar] [CrossRef]
- Johnson, M.; Lee, K.; Scow, K. DNA fingerprinting reveals links among agricultural crops, soil properties, and the composition of soil microbial communities. Geoderma 2003, 114, 279–303. [Google Scholar] [CrossRef]
- Freitas, S.S.; Santos, J.A.; Prazeres, D.M. Plasmid purification by hydrophobic interaction chromatography using sodium citrate in the mobile phase. Sep. Purif. Technol. 2009, 65, 95–104. [Google Scholar] [CrossRef]
- Xiao, F.; Chen, Z.; Wei, Z.; Tian, L. Hydrophobic Interaction: A Promising Driving Force for the Biomedical Applications of Nucleic Acids. Adv. Sci. 2020, 7, 2001048. [Google Scholar] [CrossRef]
- Katevatis, C.; Fan, A.; Klapperich, C.M. Low concentration DNA extraction and recovery using a silica solid phase. PLoS ONE 2017, 12, e0176848. [Google Scholar] [CrossRef]
- Koo, K.M.; Sina, A.A.I.; Carrascosa, L.G.; Shiddiky, M.J.A.; Trau, M. DNA–bare gold affinity interactions: Mechanism and applications in biosensing. Anal. Methods. 2015, 7, 7042–7054. [Google Scholar] [CrossRef]
- Park, J.S.; Goo, N.-I.; Kim, D.-E. Mechanism of DNA Adsorption and Desorption on Graphene Oxide. Langmuir 2014, 30, 12587–12595. [Google Scholar] [CrossRef]
- Zhai, H.; Wang, L.; Putnis, C.V. Molecular-Scale Investigations Reveal Noncovalent Bonding Underlying the Adsorption of Environmental DNA on Mica. Environ. Sci. Technol. 2019, 53, 11251–11259. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.; Mason, M.G.; Wang, Y.; Wee, E.; Turni, C.; Blackall, P.J.; Trau, M.; Botella, J.R.; Misteli, T. Nucleic acid purification from plants, animals and microbes in under 30 seconds. PLoS Biol. 2017, 15, e2003916. [Google Scholar] [CrossRef]
- Figueiredo, J.A.; Ismael, M.I.; Anjo, C.M.S.; Duarte, A.P. Cellulose and Derivatives from Wood and Fibers as Renewable Sources of Raw-Materials. In Carbohydrates in Sustainable Development I; Springer: Berlin/Heidelberg, Germany, 2010; pp. 117–128. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, H.; Shi, Z.; Liu, Y.; Liu, L.; Yu, J.; Fan, Y. Preparation of amino cellulose nanofiber via ε-poly-L-lysine grafting with enhanced mechanical, anti-microbial and food preservation performance. Ind. Crop. Prod. 2023, 194, 116288. [Google Scholar] [CrossRef]
- Mi, X.; Albukhari, S.M.; Heldt, C.L.; Heiden, P.A. Virus and chlorine adsorption onto guanidine modified cellulose nanofibers using covalent and hydrogen bonding. Carbohydr. Res. 2020, 498, 108153. [Google Scholar] [CrossRef]
- Castro, Y.; Durán, A. Control of degradation rate of Mg alloys using silica sol–gel coatings for biodegradable implant materials. J. Sol-Gel Sci. Technol. 2018, 90, 198–208. [Google Scholar] [CrossRef]
- Hui, C.-Y.; Guo, Y.; Zhang, X.; Shao, J.-H.; Yang, X.-Q.; Zhang, W. Oligodeoxyribonucleotides derived from salmon sperm DNA: An alternative to defibrotide. Biologicals 2013, 41, 190–196. [Google Scholar] [CrossRef]
- Mann, T.L.; Krull, U.J. The application of ultrasound as a rapid method to provide DNA fragments suitable for detection by DNA biosensors. Biosens. Bioelectron. 2004, 20, 945–955. [Google Scholar] [CrossRef]
- Lucena-Aguilar, G.; Sánchez-López, A.M.; Barberán-Aceituno, C.; Carrillo-Ávila, J.A.; López-Guerrero, J.A.; Aguilar-Quesada, R. DNA Source Selection for Downstream Applications Based on DNA Quality Indicators Analysis. Biopreservation Biobanking 2016, 14, 264–270. [Google Scholar] [CrossRef]
- Biradar, A.I.; Sarvalkar, P.D.; Teli, S.B.; Pawar, C.; Patil, P.; Prasad, N.R. Photocatalytic degradation of dyes using one-step synthesized silica nanoparticles. Mater. Today Proc. 2021, 43, 2832–2838. [Google Scholar] [CrossRef]
- Heptinstall, J.; Rapley, R. Spectrophotometric Analysis of Nucleic Acids. In The Nucleic Acid Protocols Handbook; Rapley, R., Ed.; Springer Protocols Handbooks: Berlin/Heidelberg, Germany; Humana Press: Totowa, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Stagi, L.; Malfatti, L.; Caboi, F.; Innocenzi, P. Thermal Induced Polymerization of l-Lysine forms Branched Particles with Blue Fluorescence. Macromol. Chem. Phys. 2021, 222, 2100242. [Google Scholar] [CrossRef]
- Shi, B.; Shin, Y.K.; Hassanali, A.A.; Singer, S.J. DNA Binding to the Silica Surface. J. Phys. Chem. B 2015, 119, 11030–11040. [Google Scholar] [CrossRef]
- Lindman, B.; Medronho, B.; Alves, L.; Norgren, M.; Nordenskiöld, L. Hydrophobic interactions control the self-assembly of DNA and cellulose. Q. Rev. Biophys. 2021, 54, e3. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rutherford, S.M.; Limson, J.; Fogel, R. Comparison of Modifications of Cellulose for the Extraction and Elution of DNA. Eng. Proc. 2025, 109, 5. https://doi.org/10.3390/engproc2025109005
Rutherford SM, Limson J, Fogel R. Comparison of Modifications of Cellulose for the Extraction and Elution of DNA. Engineering Proceedings. 2025; 109(1):5. https://doi.org/10.3390/engproc2025109005
Chicago/Turabian StyleRutherford, Shannon Megan, Janice Limson, and Ronen Fogel. 2025. "Comparison of Modifications of Cellulose for the Extraction and Elution of DNA" Engineering Proceedings 109, no. 1: 5. https://doi.org/10.3390/engproc2025109005
APA StyleRutherford, S. M., Limson, J., & Fogel, R. (2025). Comparison of Modifications of Cellulose for the Extraction and Elution of DNA. Engineering Proceedings, 109(1), 5. https://doi.org/10.3390/engproc2025109005





