In Vitro Cytotoxicity of Single Walled Carbon Nanotube Bioconjugates on Cancer Cells †
Abstract
1. Introduction
2. Materials and Methods
Cell Lines
3. Results
3.1. Heatmaps of Carbon Nanotubes and Bioconjugates
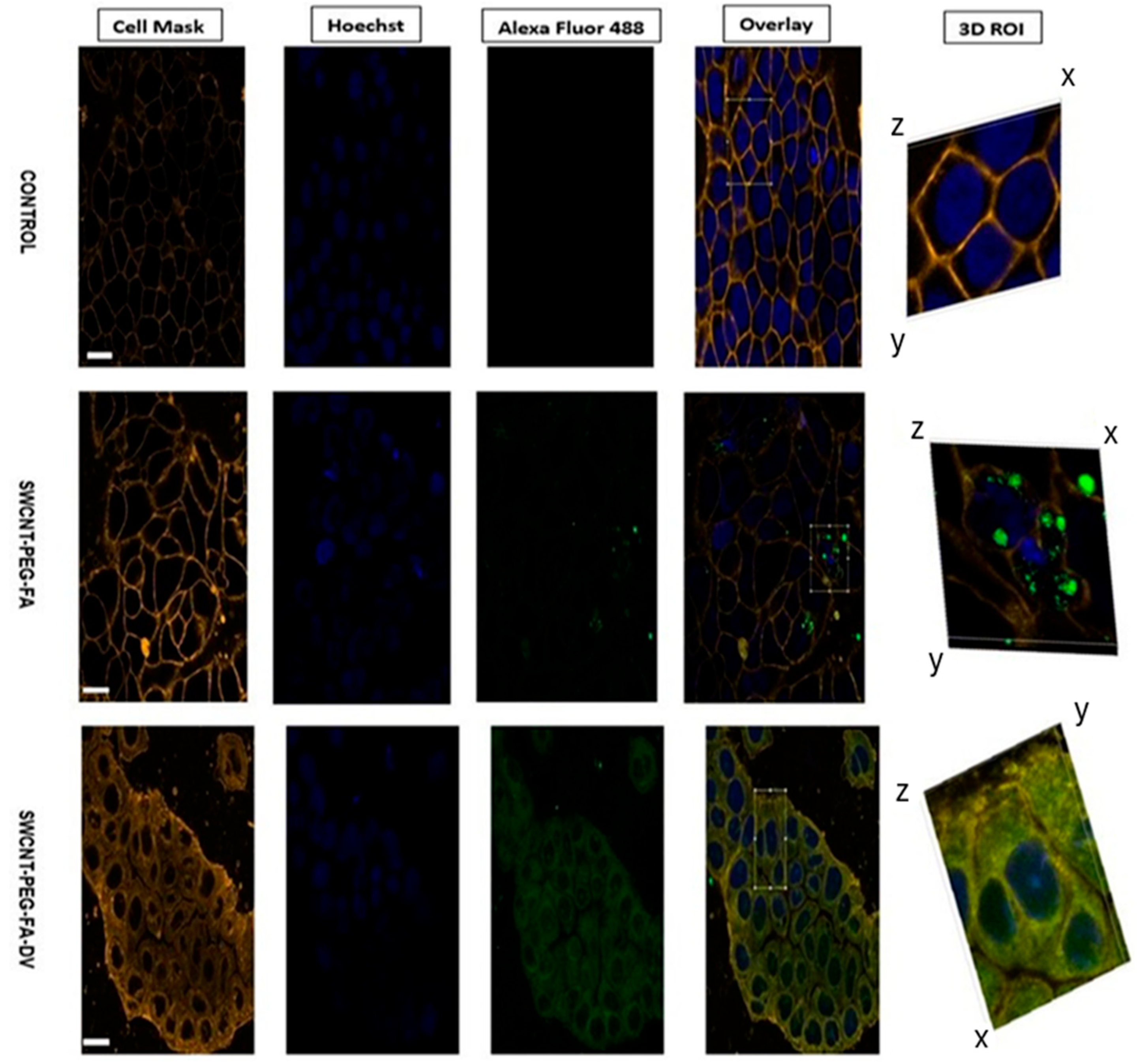
3.2. Inverted Microscopy Imaging
3.3. Fluorescence Microscopy
4. Discussion
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Astruc, D. Introduction to Nanomedicine. Molecules 2016, 21, 4. [Google Scholar] [CrossRef] [PubMed]
- Sarvarian, P.; Gholipour, E.; Asenjan, K.S.; Hojjat-Farsangi, M.; Motavalli, R.; Khiavi, F.M.; Yousefi, M. Application of emerging plant-derived nanoparticles as a novel approach for nano-drug delivery systems. Immunol. Investig. 2022, 51, 1039–1059. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Xiong, G.; Liu, Z. Toxicity of metal-based nanoparticles: Challenges in the nano era. Front. Bioeng. Biotechnol. 2022, 10, 1001572. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Zhang, Z.; Zhang, Y. The application of carbon nanotubes in target drug delivery systems for cancer therapies. Nanoscale Res. Lett. 2011, 6, 555. [Google Scholar] [CrossRef] [PubMed]
- Thiruvengadam, M.; Rajakumar, G.; Swetha, V.; Ansari, M.A.; Alghamdi, S.; Almehmadi, M.; Halawi, M.; Kungumadevi, L.; Raja, V.; Sarbudeen, S.S.; et al. Recent insights and multifactorial applications of carbon nanotubes. Micromachines 2021, 12, 1502. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.; Javeed, A.; Ahmed, W.; Phoenix, D.A.; Elhissi, A.; Jackson, M.J. Delivery of anticancer molecules using carbon nanotubes. In Surgical Tools and Medical Devices; Ahmed, W., Jackson, M.J., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 563–572. ISBN 978-3-319-33489-9. [Google Scholar] [CrossRef]
- Hafez, D.A.; Elkhodairy, K.A.; Teleb, M.; Elzoghby, A.O. Nanomedicine-based approaches for improved delivery of phyto-therapeutics for cancer therapy. Expert Opin. Drug Deliv. 2020, 17, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Abdifetah, O.; Na-Bangchang, K. Pharmacokinetic studies of nanoparticles as a delivery system for conventional drugs and herb-derived compounds for cancer therapy: A systematic review. Int. J. Nanomed. 2019, 14, 5659–5677. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gwanzura, Z.T.; Perold, W.J.; Engelbrecht, A.-M. In Vitro Cytotoxicity of Single Walled Carbon Nanotube Bioconjugates on Cancer Cells. Eng. Proc. 2025, 109, 6. https://doi.org/10.3390/engproc2025109006
Gwanzura ZT, Perold WJ, Engelbrecht A-M. In Vitro Cytotoxicity of Single Walled Carbon Nanotube Bioconjugates on Cancer Cells. Engineering Proceedings. 2025; 109(1):6. https://doi.org/10.3390/engproc2025109006
Chicago/Turabian StyleGwanzura, Zvikomborero T., Willem J. Perold, and Anna-Mart Engelbrecht. 2025. "In Vitro Cytotoxicity of Single Walled Carbon Nanotube Bioconjugates on Cancer Cells" Engineering Proceedings 109, no. 1: 6. https://doi.org/10.3390/engproc2025109006
APA StyleGwanzura, Z. T., Perold, W. J., & Engelbrecht, A.-M. (2025). In Vitro Cytotoxicity of Single Walled Carbon Nanotube Bioconjugates on Cancer Cells. Engineering Proceedings, 109(1), 6. https://doi.org/10.3390/engproc2025109006






