Emerging Trends in Paper-Based Electrochemical Biosensors for Healthcare Applications †
Abstract
1. Introduction
2. Fabrication of Paper-Based Biosensors
3. Microfluidic Integration in Paper-Based Biosensors
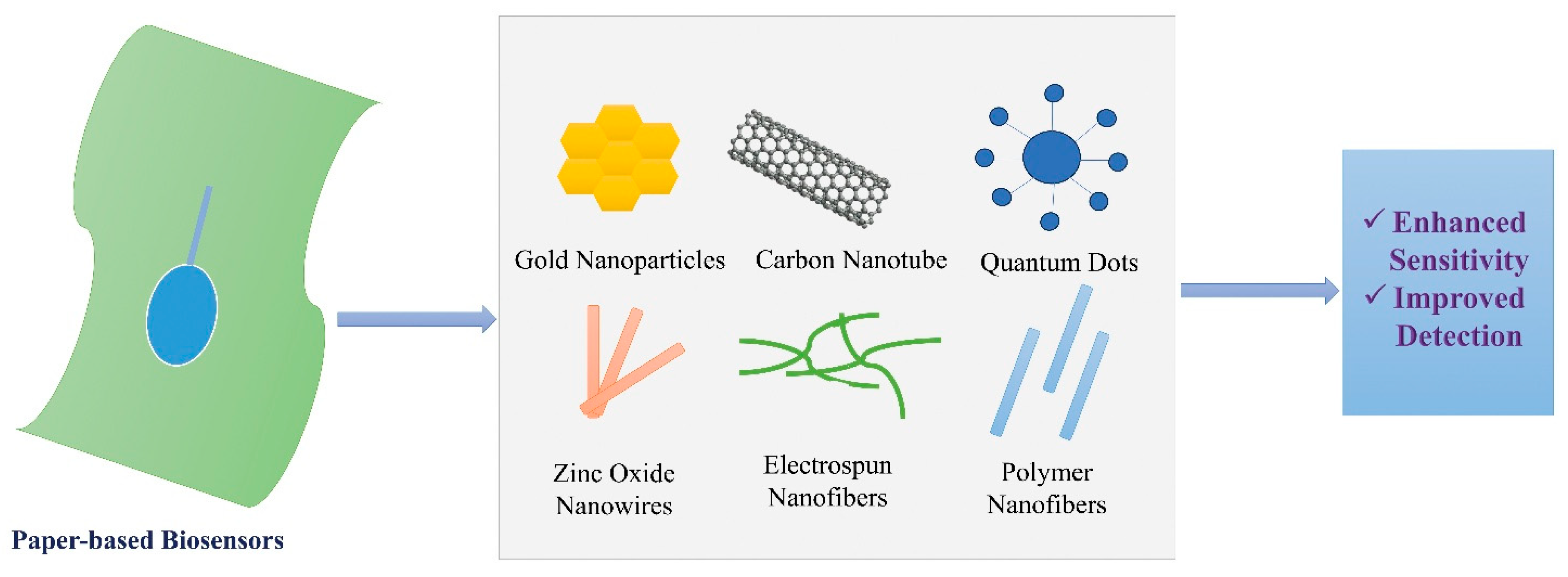
4. Nanomaterials and Surface Engineering
5. Signal Acquisition, Noise Reduction, and Amplification in Paper-Based Electrochemical Biosensors
5.1. Signal Acquisition
5.2. Noise Reduction
5.3. Signal Amplification
6. Applications in Medical Diagnostics
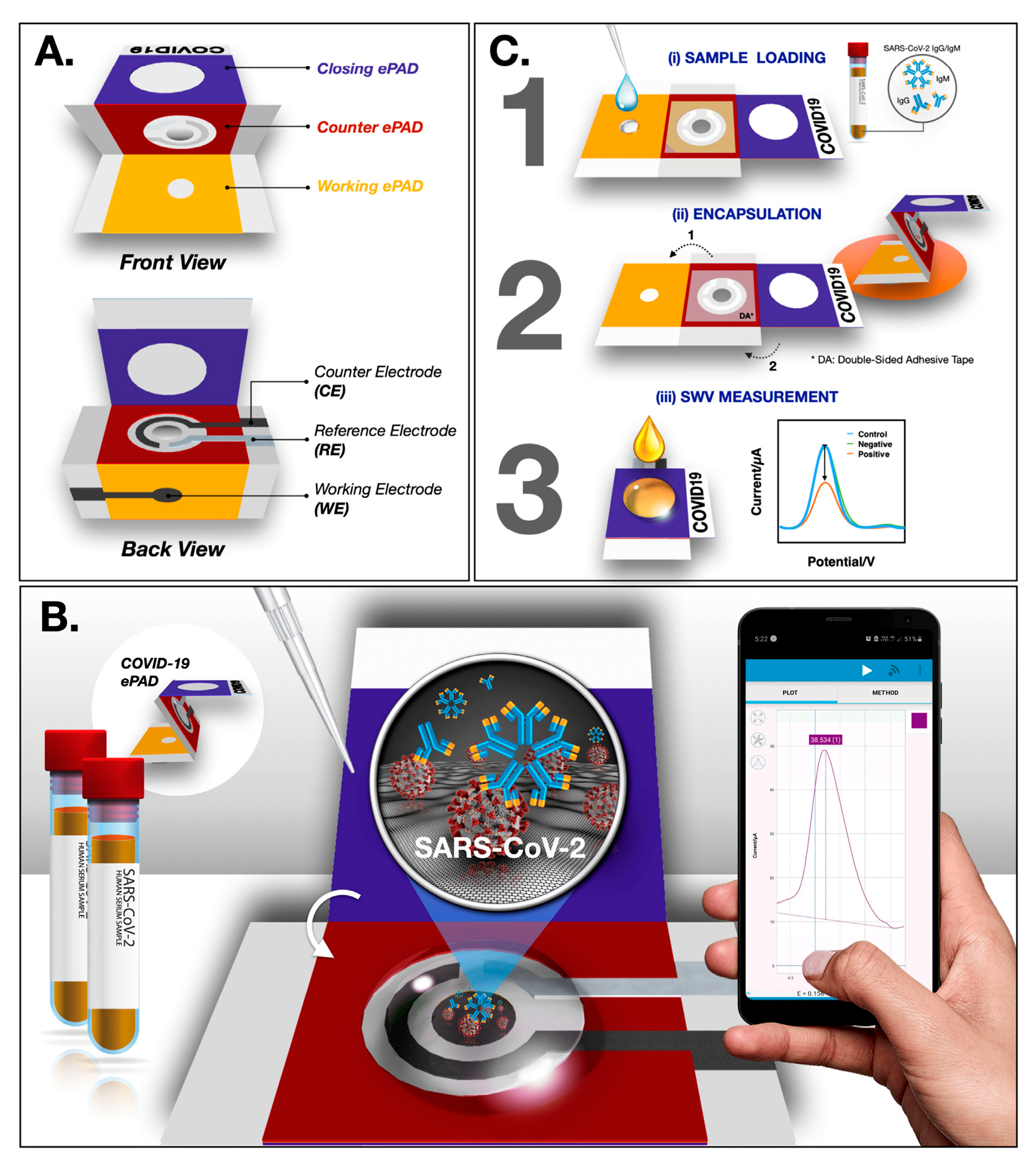
Paper-Based Biosensors for the Diagnosis of HIV, Tuberculosis (TB), COVID-19, and Malaria
7. Challenges and Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karuppannan, P.G.; Sudha, D.; Banupriya, K.; Karthik, K.; Arumugam, R. Emerging diagnostic methods using paper-based electrochemical biosensors. J. Environ. Nanotechnol. 2024, 13, 418–423. [Google Scholar] [CrossRef]
- Shrikrishna, N.S.; Sharma, R.; Gandhi, S. Paper-based biosensors: Overview from past to future. In Paper-Based Diagnostic Devices for Infectious Diseases; IOP Publishing Ltd.: Bristol, UK, 2023; pp. 1-1–1-18. [Google Scholar] [CrossRef]
- Loo, S.W.; Pui, T.-S. Cytokine and cancer biomarkers detection: The dawn of electrochemical paper-based biosensor. Sensors 2020, 20, 1854. [Google Scholar] [CrossRef] [PubMed]
- Ataide, V.N.; Pradela-Filho, L.A.; Ameku, W.A.; Negahdary, M.; Oliveira, T.G.; Santos, B.G.; Paixão, T.R.L.C.; Angnes, L. Paper-based electrochemical biosensors for the diagnosis of viral diseases. Microchim. Acta 2023, 190, 276. [Google Scholar] [CrossRef]
- Liu, B.; Du, D.; Hua, X.; Yu, X.-Y.; Lin, Y. Paper-based electrochemical biosensors: From test strips to paper-based microfluidics. Electroanalysis 2014, 26, 1214–1223. [Google Scholar] [CrossRef]
- Puiu, M.; Mirceski, V.; Bala, C. Paper-based diagnostic platforms and devices. Curr. Opin. Electrochem. 2021, 27, 100726. [Google Scholar] [CrossRef]
- Kuswandi, B.; Ensafi, A.A. Perspective—Paper-based biosensors: Trending topic in clinical diagnostics developments and commercialization. J. Electrochem. Soc. 2020, 167, 037509. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, M.; Cheng, X.; Dou, X.; Zhou, J.; Wu, J.; Xie, X.; Zhu, M. A disposable paper-based electrochemical biosensor decorated by electrospun cellulose acetate nanofibers for highly sensitive bio-detection. Analyst 2024, 149, 2436–2444. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, S.; Zhang, Z.; Yang, Z.; Cui, X.; Liu, G. Printable biosensors towards next-generation point-of-care testing: Paper substrate as an example. Lab A Chip 2023, 23, 3328–3352. [Google Scholar] [CrossRef]
- Li, Z.; Li, F.; Xing, Y.; Liu, Z.; You, M.; Li, Y.; Wen, T.; Qu, Z.; Ling Li, X.; Xu, F. Pen-on-paper strategy for point-of-care testing: Rapid prototyping of fully written microfluidic biosensor. Biosens. Bioelectron. 2017, 98, 478–485. [Google Scholar] [CrossRef]
- Ozoglu, O.; Korukluoglu, M.; Uzunoglu, A. Electrochemical detection of foodborne Escherichia coli using carbon nanotube-incorporated pencil-drawn paper-based disposable biosensors. Anal. Chim. Acta 2025, 1369, 344344. [Google Scholar] [CrossRef]
- Ma, P.; Wang, S.; Wang, J.; Wang, Y.; Dong, Y.; Li, S.; Su, H.; Chen, P.; Feng, X.; Li, Y.; et al. Rapid assembly of cellulose microfibers into translucent and flexible microfluidic paper-based analytical devices via wettability patterning. Anal. Chem. 2022, 94, 13332–13341. [Google Scholar] [CrossRef]
- Aydın, E.B.; Aydın, M.; Sezgintürk, M.K. Paper-based devices. In Commercial Biosensors and Their Applications: Clinical, Food, and Beyond; Elsevier: Amsterdam, The Netherlands, 2020; pp. 107–166. [Google Scholar] [CrossRef]
- Bilgen, E.; Suvacı, Z.; Persil Çetinkol, Ö.; Forough, M. Disposable paper-based sensors. In Fundamentals of Sensor Technology: Principles and Novel Designs; Woodhead Publishing: London, UK, 2023; pp. 803–860. [Google Scholar] [CrossRef]
- Dillon, M.J.; Campbell, K. Hyphenating paper-based biosensors with smartphones. In Comprehensive Analytical Chemistry; Elsevier: Amsterdam, The Netherlands, 2023; Volume 101, pp. 109–141. [Google Scholar] [CrossRef]
- Huang, J.; Pan, J.; Song, Y.; Lin, Q.; Xu, Y.; Dai, Z.; Liu, S.-Y. MOF-functionalized paper-based biosensors: Fabrications, mechanisms and applications. TrAC—Trends Anal. Chem. 2024, 173, 117619. [Google Scholar] [CrossRef]
- Torul, H.; Yarali, E.; Eksin, E.; Ganguly, A.; Benson, J.; Tamer, U.; Papakonstantinou, P.; Erdem, A. Paper-based electrochemical biosensors for voltammetric detection of miRNA biomarkers using reduced graphene oxide or MoS2 nanosheets decorated with gold nanoparticle electrodes. Biosensors 2021, 11, 236. [Google Scholar] [CrossRef]
- Somarathna, K.U.S.; Garakani, B.; Weerawarne, D.L.; Khinda, G.S.; Burns, A.; Alizadeh, A.; Poliks, M.D. Screen-printed water-soluble resistors for wearable electronics: An analysis of the fabrication process. In Proceedings of the Electronic Components and Technology Conference, San Diego, CA, USA, 1 June–4 July 2021; pp. 2285–2292. [Google Scholar] [CrossRef]
- Kołodziej, L.; Ostrowski, S.; Maciejewski, A.; Jakubowska, M.; Wróblewski, G. The influence of screen-printing parameters on properties of conductive layers for application in biomedical electrodes. In Advances in Intelligent Systems and Computing; Springer: Berlin/Heidelberg, Germany, 2020; Volume 1044, pp. 406–413. [Google Scholar] [CrossRef]
- Campos-Arias, L.; Peřinka, N.; Lau, Y.C.; Castro, N.; Pereira, N.; Correia, V.M.G.; Costa, P.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. Improving definition of screen-printed functional materials for sensing application. ACS Appl. Electron. Mater. 2024, 6, 2152–2160. [Google Scholar] [CrossRef]
- Ali, M.; Lin, L.; Faisal, S.; Sahito, I.A.; Ali, S.I. Optimisation of screen printing process for functional printing. Pigment Resin Technol. 2019, 48, 456–463. [Google Scholar] [CrossRef]
- Pan, J.; Keif, M.; Ledgerwood, J.; Rong, X.; Wang, X. Screen printing fine pitch stretchable silver inks onto a flexible substrate for wearable applications. J. Microelectron. Electron. Packag. 2018, 15, 179–186. [Google Scholar] [CrossRef]
- Kuo, C.-C.; You, Z.-Y. Development of injection molding tooling with conformal cooling channels fabricated by optimal process parameters. Int. J. Adv. Manuf. Technol. 2018, 96, 1003–1013. [Google Scholar] [CrossRef]
- Prianto, E.; Herianto; Herliansyah, M.K. Optimization of wax model printing using a DLP 3D printer for castable wax resin material. E3S Web Conf. 2024, 517, 05022. [Google Scholar] [CrossRef]
- Yadav, A.C.; Navin Kumar, N.; Raja, K.; Naiju, C.D. Investigations on Dimensional Analysis of Fused Filament Fabrication of Wax Filament by Taguchi Design; SAE Technical Papers; SAE International: Warrendale, PA, USA, 2019. [Google Scholar] [CrossRef]
- Yan, Y.; Huang, X.; Yuan, L.; Tang, Y.; Zhu, W.; Du, H.; Nie, J.; Zhang, L.; Liao, S.; Tang, X.; et al. Single-step batch fabrication of microfluidic paper-based analytical devices with a 3D printer and their applications in nanoenzyme-enhanced visual detection of dopamine. Anal. Bioanal. Chem. 2024, 416, 4131–4141. [Google Scholar] [CrossRef] [PubMed]
- Qamar, A.Z.; Amar, K.; Kohli, P.; Chowdhury, F.; Shamsi, M.H. Wax patterned microwells for stem cell fate study. RSC Adv. 2016, 6, 104919–104924. [Google Scholar] [CrossRef]
- Cui, X.-J.; Lian, J.; Zhang, X.-P. Process of investment casting wax pattern formed by fused deposition modeling based on finite element method. Zhuzao/Foundry 2020, 69, 1203–1206. Available online: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85103447717&partnerID=40&md5=70898bdcd90124e5d4987e5c54cebdce (accessed on 15 January 2025).
- Colozza, N.; Caratelli, V.; Moscone, D.; Arduini, F. Origami paper-based electrochemical (Bio)sensors: State of the art and perspective. Biosensors 2021, 11, 328. [Google Scholar] [CrossRef]
- Dong, X.; Song, P.; Liu, X. Rapid prototyping of paper-based electronics by robotic printing and micromanipulation. In Proceedings of the IEEE International Conference on Automation Science and Engineering (CASE), Xi’an, China, 20–23 August 2017; pp. 586–591. [Google Scholar] [CrossRef]
- Yang, M.; Liu, M.; Cheng, J.; Wang, H. A movable type bioelectronics printing technology for modular fabrication of biosensors. Sci. Rep. 2021, 11, 22323. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cao, R.; Wu, J.; Guan, L.; Li, M.; Liu, J.; Tian, J. Directly writing barrier-free patterned biosensors and bioassays on paper for low-cost diagnostics. Sens. Actuators B Chem. 2019, 285, 529–535. [Google Scholar] [CrossRef]
- Gao, P.; Kasama, T.; Shin, J.; Huang, Y.; Miyake, R. A mediated enzymatic electrochemical sensor using paper-based laser-induced graphene. Biosensors 2022, 12, 995. [Google Scholar] [CrossRef]
- Gandhiraman, R.P.; Nordlund, D.; Jayan, V.; Meyyappan, M.; Koehne, J.E. Scalable low-cost fabrication of disposable paper sensors for DNA detection. ACS Appl. Mater. Interfaces 2014, 6, 22751–22760. [Google Scholar] [CrossRef]
- Cagnani, G.R.; Ibáñez-Redín, G. Fundamentals concepts of the large-scale deposition techniques applied to biodevices manufacturing. In Advances in Bioelectrochemistry Volume 2: Biomimetic, Bioelectrocatalysis and Materials Interfaces; Springer: Berlin/Heidelberg, Germany, 2022; pp. 55–70. [Google Scholar] [CrossRef]
- Cheng, Q.; Zhang, J.; Wang, H.; Wang, D.; Feng, X.; Jiang, L. High-performance flexible bioelectrocatalysis bioassay system based on a triphase interface. Adv. Mater. Interfaces 2020, 7, 1902172. [Google Scholar] [CrossRef]
- Castro, L.F.; Silva-Neto, H.A.; Sousa, L.R.; de Araujo, W.R.; Coltro, W.K.T. Silicone glue-based graphite ink incorporated on paper platform as an affordable approach to construct stable electrochemical sensors. Talanta 2022, 251, 123812. [Google Scholar] [CrossRef]
- Zhao, C.; Thuo, M.M.; Liu, X. A microfluidic paper-based electrochemical biosensor array for multiplexed detection of metabolic biomarkers. Sci. Technol. Adv. Mater. 2013, 14, 054402. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, X. A paper-based microfluidic device for multiplexed electrochemical detection of biomarkers. In Proceedings of the 2013 International Conference on Manipulation, Manufacturing and Measurement on the Nanoscale, 3M-NANO 2013, Suzhou, China, 26–30 August 2013; pp. 162–165. [Google Scholar] [CrossRef]
- Hasanzadeh, A.; Hashemzadeh, I. Microfluidic paper-based devices. In Biomedical Applications of Microfluidic Devices; Elsevier: Amsterdam, The Netherlands, 2020; pp. 257–274. [Google Scholar] [CrossRef]
- Vereshchagina, E. Paper microfluidics. In Microfluidics for Biologists: Fundamentals and Applications; Springer: Berlin/Heidelberg, Germany, 2016; pp. 165–190. [Google Scholar] [CrossRef]
- Wang, S.; Guan, X.; Sun, S. Microfluidic biosensors: Enabling advanced disease detection. Sensors 2025, 25, 1936. [Google Scholar] [CrossRef]
- Limbut, W.; Promsuwan, K.; Kongkaew, S.; Thavarungkul, P.; Mak, W.C. Emerging functional materials for microfluidic biosensors. In Microfluidic Biosensors; Elsevier: Amsterdam, The Netherlands, 2022; pp. 195–231. [Google Scholar] [CrossRef]
- Kumar, R.; Chauhan, G.; Martinez-Chapa, S.O. Metal oxides and their composites as flow-through biosensors for biomonitoring. In Metal Oxides for Biomedical and Biosensor Applications; Elsevier: Amsterdam, The Netherlands, 2021; pp. 291–319. [Google Scholar] [CrossRef]
- Gabriel, E.F.M.; Garcia, P.T.; Evans, E.; Cardoso, T.M.G.; Garcia, C.D.; Coltro, W.K.T. Enhanced performance of colorimetric biosensing on paper microfluidic platforms through chemical modification and incorporation of nanoparticles. In Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2017; Volume 1571, pp. 327–341. [Google Scholar] [CrossRef]
- Zhu, J.; Hoettges, K.; Wang, Y.; Ma, H.; Song, P.; Hu, Y.; Lim, E.G.; Zhang, Q. TimePAD—Unveiling temporal sequence ELISA signal by deep learning for rapid readout and improved accuracy in a microfluidic paper-based analytical platform. Anal. Chem. 2025, 97, 4515–4523. [Google Scholar] [CrossRef]
- Jia, Y.; Sun, H.; Dong, H.; Wang, C.; Lin, X.; Dong, D. Scalable and parallelized biochemical assays in paper devices integrated with a programmable binary valve matrix. Sens. Actuators B Chem. 2020, 321, 128466. [Google Scholar] [CrossRef]
- Suvanasuthi, R.; Chimnaronk, S.; Promptmas, C. 3D printed hydrophobic barriers in a paper-based biosensor for point-of-care detection of dengue virus serotypes. Talanta 2022, 237, 122962. [Google Scholar] [CrossRef]
- Lim, H.; Jafry, A.T.; Lee, J. Fabrication, flow control, and applications of microfluidic paper-based analytical devices. Molecules 2019, 24, 2869. [Google Scholar] [CrossRef] [PubMed]
- Tanjaya, H.; Harito, C. Integrating microfluidic and biosensors: A mini review. J. Phys. Conf. Ser. 2024, 2705, 012018. [Google Scholar] [CrossRef]
- Chen, D.; Chen, N.; Liu, F.; Wang, Y.; Liang, H.; Yang, Y.; Yuan, Q. Flexible point-of-care electrodes for ultrasensitive detection of bladder tumor-relevant miRNA in urine. Anal. Chem. 2023, 95, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Islam, M.; Gupta, A. Paper-based multiplex biosensors for inexpensive healthcare diagnostics: A comprehensive review. Biomed. Microdevices 2023, 25, 17. [Google Scholar] [CrossRef]
- Valero, M.; De Leon, I.; Ray, M.; Berini, P. Multiplexed biosensors based on interference of surface plasmons in multimode nanoslits. Appl. Opt. 2025, 64, 50–63. [Google Scholar] [CrossRef]
- Guo, X.; Zong, L.; Jiao, Y.; Han, Y.; Zhang, X.; Xu, J.; Li, L.; Zhang, C.-W.; Liu, Z.; Ju, Q.; et al. Signal-enhanced detection of multiplexed cardiac biomarkers by a paper-based fluorogenic immunodevice integrated with zinc oxide nanowires. Anal. Chem. 2019, 91, 9300–9307. [Google Scholar] [CrossRef]
- Tian, R.; Li, Y.; Bai, J. Hierarchical assembled nanomaterial paper-based analytical devices for simultaneously electrochemical detection of microRNAs. Anal. Chim. Acta 2019, 1058, 89–96. [Google Scholar] [CrossRef]
- Wang, F.; Liu, Y.; Fu, C.; Li, N.; Du, M.; Zhang, L.; Ge, S.; Yu, J. Paper-based bipolar electrode electrochemiluminescence platform for detection of multiple miRNAs. Anal. Chem. 2021, 93, 1702–1708. [Google Scholar] [CrossRef]
- Lee, Y.; Ma, H.; Jun, B.-H.; Kim, S.-J. Automated paper-based immunoassay device for ultrasensitive multibiomarker detection with an integrated mechanical timer actuator. Sens. Actuators B Chem. 2025, 442, 138099. [Google Scholar] [CrossRef]
- Wu, Y.; Xue, P.; Hui, K.M.; Kang, Y. A paper-based microfluidic electrochemical immunodevice integrated with amplification-by-polymerization for the ultrasensitive multiplexed detection of cancer biomarkers. Biosens. Bioelectron. 2014, 52, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Zong, L.; Han, Y.; Gao, L.; Du, C.; Zhang, X.; Li, L.; Huang, X.; Liu, J.; Yu, H.-D.; Huang, W. A transparent paper-based platform for multiplexed bioassays by wavelength-dependent absorbance/transmittance. Analyst 2019, 144, 7157–7161. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Tran, T.-T.; Modha, S.; Tsutsui, H.; Mulchandani, A. A paper-based chemiresistive biosensor employing single-walled carbon nanotubes for low-cost, point-of-care detection. Biosens. Bioelectron. 2019, 130, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Caratelli, V.; Di Meo, E.; Colozza, N.; Fabiani, L.; Fiore, L.; Moscone, D.; Arduini, F. Nanomaterials and paper-based electrochemical devices: Merging strategies for fostering sustainable detection of biomarkers. J. Mater. Chem. B 2022, 10, 9021–9039. [Google Scholar] [CrossRef]
- Shi, K.; Yi, Z.; Han, Y.; Chen, J.; Hu, Y.; Cheng, Y.; Liu, S.; Wang, W.; Song, J. PAM-free cascaded strand displacement coupled with CRISPR-Cas12a for amplified electrochemical detection of SARS-CoV-2 RNA. Anal. Biochem. 2023, 664, 115046. [Google Scholar] [CrossRef]
- Haarindra Prasad, R.P.; Hashim, U. A review on the label free nanowire based biosensor. J. Appl. Sci. Res. 2012, 8, 4759–4769. [Google Scholar]
- Seddaoui, N.; Colozza, N.; Gullo, L.; Arduini, F. Paper as smart support for bioreceptor immobilization in electrochemical paper-based devices. Int. J. Biol. Macromol. 2023, 253, 127409. [Google Scholar] [CrossRef]
- Chalupniak, A.; Merkoçi, A. Recent trends in nanomaterials integration into simple biosensing platforms. In Nanotechnology in Biology and Medicine: Methods, Devices, and Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2017; pp. 389–405. [Google Scholar] [CrossRef]
- Siebe, H.S.; Chen, Q.; Li, X.; Xu, Y.; Browne, W.R.; Bell, S.E.J. Filter paper based SERS substrate for the direct detection of analytes in complex matrices. Analyst 2021, 146, 1281–1288. [Google Scholar] [CrossRef]
- Thirugnanasambandan, T. Functionalization on sensing surfaces for efficient biomolecular capturing. In Nanobiosensors for Biomolecular Targeting; Elsevier: Amsterdam, The Netherlands, 2018; pp. 51–94. [Google Scholar] [CrossRef]
- Rocha, J.F.; de Oliveira, J.C.; Bettini, J.; Strauss, M.; Selmi, G.S.; Okazaki, A.K.; de Oliveira, R.F.; Lima, R.S.; Santhiago, M. Tuning the chemical and electrochemical properties of paper-based carbon electrodes by pyrolysis of polydopamine. ACS Meas. Sci. Au 2024, 4, 188–200. [Google Scholar] [CrossRef]
- Roy, L.; Buragohain, P.; Borse, V. Strategies for sensitivity enhancement of point-of-care devices. Biosens. Bioelectron. X 2022, 10, 100098. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Shadjou, N. Electrochemical and photoelectrochemical nano-immunesensing using origami paper based method. Mater. Sci. Eng. C 2016, 61, 979–1001. [Google Scholar] [CrossRef]
- Rahman, M.A.; Pal, R.K.; Islam, N.; Freeman, R.; Berthiaume, F.; Mazzeo, A.; Ashraf, A. A facile graphene conductive polymer paper based biosensor for dopamine, TNF-α, and IL-6 detection. Sensors 2023, 23, 8115. [Google Scholar] [CrossRef]
- Hosseini, Z.; Yuan, G.J. Flexible electrochemical biosensor with graphene and gold nanoparticle modification for enhanced e-ELISA point-of-care biomarker detection. In 2024 IEEE Sensors; IEEE: Piscataway, NJ, USA, 2024. [Google Scholar] [CrossRef]
- Hunt, A.; Torati, S.R.; Slaughter, G. Paper-based DNA biosensor for rapid and selective detection of miR-21. Biosensors 2024, 14, 485. [Google Scholar] [CrossRef]
- Rai, M.; Nguyen, T.A. Nanomaterials Recycling; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar] [CrossRef]
- Holder, A.L.; Vejerano, E.P.; Zhou, X.; Marr, L.C. Nanomaterial disposal by incineration. Environ. Sci. Process. Impacts 2013, 15, 1652–1664. [Google Scholar] [CrossRef] [PubMed]
- Pandey, J.K.; Bobde, P.; Patel, R.K.; Manna, S. Disposal and Recycling Strategies for Nano-Engineered Materials; Elsevier: Amsterdam, The Netherlands, 2023. [Google Scholar] [CrossRef]
- Gupta, Y.; Sharma, A.; Pandey, C.M. Nanopapers-based biosensors for point-of-care diagnostics. In Handbook of Nanobioelectrochemistry: Application in Devices and Biomolecular Sensing; Springer: Berlin/Heidelberg, Germany, 2023; pp. 383–411. [Google Scholar] [CrossRef]
- Saravanan, J.; Nair, A.; Krishna, S.S.; Viswanad, V. Nanomaterials in biology and medicine: A new perspective on its toxicity and applications. Drug Chem. Toxicol. 2024, 47, 767–784. [Google Scholar] [CrossRef] [PubMed]
- Vankayala, R.; Kalluru, P.; Tsai, H.-H.; Chiang, C.-S.; Hwang, K.C. Effects of surface functionality of carbon nanomaterials on short-term cytotoxicity and embryonic development in zebrafish. J. Mater. Chem. B 2014, 2, 1038–1047. [Google Scholar] [CrossRef] [PubMed]
- Langari, M.M.; Nikzad, M.; Labidi, J. Nanocellulose-based sensors in medical/clinical applications: The state-of-the-art review. Carbohydr. Polym. 2023, 304, 120509. [Google Scholar] [CrossRef]
- González-Gálvez, D.; Janer, G.; Vilar, G.; Vílchez, A.; Vázquez-Campos, S. The life cycle of engineered nanoparticles. In Advances in Experimental Medicine and Biology; Demidov, A.V., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 947, pp. 41–69. [Google Scholar] [CrossRef]
- Gallagher, M.J.; Allen, C.; Buchman, J.T.; Qiu, T.A.; Clement, P.L.; Krause, M.O.P.; Gilbertson, L.M. Research highlights: Applications of life-cycle assessment as a tool for characterizing environmental impacts of engineered nanomaterials. Environ. Sci. Nano 2017, 4, 276–281. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, C.; Xi, F.; Chen, W.; Jiao, K.; Du, Q.; Bai, F.; Liu, Z. Experimental study on the influence of environment conditions on the performance of paper-based microfluidic fuel cell. Appl. Therm. Eng. 2023, 219, 119487. [Google Scholar] [CrossRef]
- Salehnezhad, Z.; Soroosh, M.; Mondal, H. A Highly Sensitive Plasmonic Graphene-Based Structure for Deoxyribonucleic Acid Detection. Photonics 2024, 11, 549. [Google Scholar] [CrossRef]
- Wawrzynek, E.; Baumbauer, C.; Arias, A.C. Characterization and comparison of biodegradable printed capacitive humidity sensors. Sensors 2021, 21, 6557. [Google Scholar] [CrossRef]
- Allarà, C.; Vasquez, S.; Ahmad, M.; Ibba, P.; Angeli, M.A.C.; Altana, A.; Rivadeneyra, A.; Benson, N.; Lugli, P.; Petti, L. Temperature sensor fabricated via direct laser writing on copper nanoparticles coated paper substrates. In Proceedings of the IEEE Sensors, Kobe, Japan, 20–23 October 2024. [Google Scholar] [CrossRef]
- Nota, G.; Cimmino, W.; Singh, S.; Darwish, I.A.; La Rocca, C.; Carbone, F.; Matarese, G.; Cinti, S. A portable and ecological paper-based device for glucose monitoring in peripheral blood mononuclear cell lysates. Anal. Methods 2025, 17, 2529–2535. [Google Scholar] [CrossRef]
- Papadimitriou, K.I.; Evans, D.; Morgan, H.; Prodromakis, T. A PCB-based electronic ELISA system for rapid, portable infectious disease diagnosis. In Proceedings of the 2016 IEEE Biomedical Circuits and Systems Conference (BioCAS 2016), Shanghai, China, 17–19 October 2016; pp. 252–255. [Google Scholar] [CrossRef]
- Wu, Y.; Xue, P.; Kang, Y.; Hui, K.M. Paper-based microfluidic electrochemical immunodevice integrated with nanobioprobes onto graphene film for ultrasensitive multiplexed detection of cancer biomarkers. Anal. Chem. 2013, 85, 8661–8668. [Google Scholar] [CrossRef] [PubMed]
- Campàs, M.; Olteanu, M.G.; Marty, J.-L. Enzymatic recycling for signal amplification: Improving microcystin detection with biosensors. Sens. Actuators B Chem. 2008, 129, 263–267. [Google Scholar] [CrossRef]
- Zhou, C.; Cui, K.; Liu, Y.; Hao, S.; Zhang, L.; Ge, S.; Yu, J. Ultrasensitive microfluidic paper-based electrochemical/visual analytical device via signal amplification of Pd@hollow Zn/Co core–shell ZIF67/ZIF8 nanoparticles for prostate-specific antigen detection. Anal. Chem. 2021, 93, 5459–5467. [Google Scholar] [CrossRef]
- Baharfar, M.; Rahbar, M.; Tajik, M.; Liu, G. Engineering strategies for enhancing the performance of electrochemical paper-based analytical devices. Biosens. Bioelectron. 2020, 167, 112506. [Google Scholar] [CrossRef]
- Rahul, P.K.; Kummari, S.; Krishnan, S. Signal amplification strategies for biosensing of clinically important analytes. In Biosensors for Personalized Healthcare; Springer: Berlin/Heidelberg, Germany, 2024; pp. 115–193. [Google Scholar] [CrossRef]
- Settu, K.; Huang, Y.-M.; Zhou, S.-X. A facile approach for the electrochemical sensing of dopamine using paper-based PEDOT:PSS/RGO graphene biosensor. ECS J. Solid State Sci. Technol. 2020, 9, 121002. [Google Scholar] [CrossRef]
- Ataide, V.N.; Arantes, I.V.S.; Mendes, L.F.; Rocha, D.S.; Baldo, T.A.; Coltro, W.K.T.; Paixão, T.R.L.C. Review—A pencil drawing overview: From graphite to electrochemical sensors/biosensors applications. J. Electrochem. Soc. 2022, 169, 047524. [Google Scholar] [CrossRef]
- Cinti, S.; Minotti, C.; Moscone, D.; Palleschi, G.; Arduini, F. Fully integrated ready-to-use paper-based electrochemical biosensor to detect nerve agents. Biosens. Bioelectron. 2017, 93, 46–51. [Google Scholar] [CrossRef]
- Benjamin, S.R.; de Lima, F.; Nascimento, V.A.D.; de Andrade, G.M.; Oriá, R.B. Advancement in paper-based electrochemical biosensing and emerging diagnostic methods. Biosensors 2023, 13, 689. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.B.C.; Mohd-Naim, N.F.; Tamiya, E.; Ahmed, M.U. Trends in paper-based electrochemical biosensors: From design to application. Anal. Sci. 2018, 34, 7–18. [Google Scholar] [CrossRef]
- Pingarrón, J.M.; Yáñez-Sedeño, P.; Campuzano, S. New tools of electrochemistry at the service of (bio)sensing: From rational designs to electrocatalytic mechanisms. J. Electroanal. Chem. 2021, 896, 115097. [Google Scholar] [CrossRef]
- Umar, L.; Setiadi, R.N.; Arfianti, A. Noise reduction of amperometric electrochemical sensor using current compensation on transimpedance amplifier for dissolved oxygen measurement. Fluct. Noise Lett. 2025, 24, 2550015. [Google Scholar] [CrossRef]
- Maleki, M.J.; Soroosh, M.; AL-Shammri, F.K.; Alkhayer, A.G.; Mondal, H. Design and simulation of a compact subwavelength graphene-based switch for surface plasmon polariton transmission in integrated optoelectronic circuits. Plasmonics 2025, 20, 3605–3617. [Google Scholar] [CrossRef]
- Bednar, T.; Babusiak, B.; Labuda, M.; Smetana, M.; Borik, S. Common-mode voltage reduction in capacitive sensing of biosignal using capacitive grounding and DRL electrode. Sensors 2021, 21, 2568. [Google Scholar] [CrossRef]
- Larsen, S.T.; Heien, M.L.; Taboryski, R. Amperometric noise at thin film band electrodes. Anal. Chem. 2012, 84, 7744–7749. [Google Scholar] [CrossRef] [PubMed]
- Aviles-Espinosa, R.; Dore, H.; Rendon-Morales, E. An experimental method for bio-signal denoising using unconventional sensors. Sensors 2023, 23, 3527. [Google Scholar] [CrossRef]
- Hashkavayi, A.B.; Raoof, J.B. Nucleic acid-based electrochemical biosensors. In Electrochemical Biosensors; Elsevier: Amsterdam, The Netherlands, 2019; pp. 253–276. [Google Scholar] [CrossRef]
- Pornprom, T.; Phusi, N.; Thongdee, P.; Pakamwong, B.; Sangswan, J.; Kamsri, P.; Punkvang, A.; Suttisintong, K.; Leanpolchareanchai, J.; Hongmanee, P.; et al. Toward the early diagnosis of tuberculosis: A gold particle-decorated graphene-modified paper-based electrochemical biosensor for Hsp16.3 detection. Talanta 2024, 267, 125210. [Google Scholar] [CrossRef] [PubMed]
- Antiochia, R. Paper-based biosensors: Frontiers in point-of-care detection of COVID-19 disease. Biosensors 2021, 11, 110. [Google Scholar] [CrossRef] [PubMed]
- Geldert, A.; Kenry; Lim, C.T. Paper-based MoS2 nanosheet-mediated FRET aptasensor for rapid malaria diagnosis. Sci. Rep. 2017, 7, 17510. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, F.; Hu, H.; Zhang, Z.; Yang, H.; Yao, X.; Liu, H.; Zheng, J. A paper-based label-free plasmonic nanosensor for portable pre-diagnosis of multiple metabolic diseases. Biosens. Bioelectron. 2025, 275, 117231. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, P.; Arora, B.; Saxena, S.; Singh, S.; Palkar, P.; Goda, J.S.; Banerjee, R. Paper-based point of care diagnostics for cancer biomarkers. Sens. Diagn. 2024, 3, 504–535. [Google Scholar] [CrossRef]
- Yakoh, A.; Pimpitak, U.; Rengpipat, S.; Hirankarn, N.; Chailapakul, O.; Chaiyo, S. Paper-based electrochemical biosensor for diagnosing COVID-19: Detection of SARS-CoV-2 antibodies and antigen. Biosens. Bioelectron. 2021, 176, 112912. [Google Scholar] [CrossRef]
- Loynachan, C.N.; Thomas, M.R.; Gray, E.R.; Richards, D.A.; Kim, J.; Miller, B.S.; Brookes, J.C.; Agarwal, S.; Chudasama, V.; McKendry, R.A.; et al. Platinum nanocatalyst amplification: Redefining the gold standard for lateral flow immunoassays with ultrabroad dynamic range. ACS Nano 2018, 12, 279–288. [Google Scholar] [CrossRef]
- Adedokun, G.; Sidhu, G.; Wang, G.P.; Hugh Fan, Z. Development of paper-based RNA amplification devices for point-of-care detection of HIV. In Proceedings of the ASME International Mechanical Engineering Congress and Exposition, Proceedings (IMECE), New Orleans, LA, USA, 29 October–2 November 2023; 5p. [Google Scholar] [CrossRef]
- Rotake, D.R.; Zalke, J.B.; Anjankar, S.C.; Singh, S.G. A novel chemiresistive biosensor utilizing green-synthesized AgNPs and MWCNT-ZnO nanofibers for rapid detection of tuberculosis Lipoarabinomannan (LAM) antigen. Anal. Chim. Acta 2025, 1358, 344092. [Google Scholar] [CrossRef]
- Sundaram, K.; Subramani, S.; Prabhu, V. Nanoparticle-based biosensor—From existing to advanced tools in diagnosis of tuberculosis. Indian J. Tuberc. 2025, in press. [Google Scholar] [CrossRef]
- Seo, G.; Lee, G.; Kim, W.; An, I.; Choi, M.; Jang, S.; Park, Y.-J.; Lee, J.-O.; Cho, D.; Park, E.C. Ultrasensitive biosensing platform for Mycobacterium tuberculosis detection based on functionalized graphene devices. Front. Bioeng. Biotechnol. 2023, 11, 1313494. [Google Scholar] [CrossRef]
- Rasmi, Y.; Li, X.; Khan, J.; Ozer, T.; Choi, J.R. Emerging point-of-care biosensors for rapid diagnosis of COVID-19: Current progress, challenges, and future prospects. Anal. Bioanal. Chem. 2021, 413, 4137–4159. [Google Scholar] [CrossRef]
- Ogunmolasuyi, A.M.; Fogel, R.; Hoppe, H.; Goldring, D.; Limson, J. A microfluidic paper analytical device using capture aptamers for the detection of PfLDH in blood matrices. Malar. J. 2022, 21, 174. [Google Scholar] [CrossRef] [PubMed]
- Siriyod, N.; Prabowo, M.H.; Cheeveewattanagul, N.; Manopwisedjaroen, K.; Nguitragool, W.; Sattabongkot Prachumsri, J.; Surareungchai, W.; Rijiravanich, P. Microfluidic paper-based analytical device for point-of-care nucleic acid quantification of malaria. Microchem. J. 2025, 212, 113139. [Google Scholar] [CrossRef]
- Phan, L.M.T.; Hoang, T.X.; Vo, T.A.T.; Kim, J.Y.; Lee, S.-M.; Cho, W.W.; Kim, Y.H.; Choi, S.H.; Cho, S. Nanobiosensors for non-amyloidbeta-tau biomarkers as advanced reporters of Alzheimer’s disease. Diagnostics 2020, 10, 913. [Google Scholar] [CrossRef]
- Pereira, M.V.; Marques, A.C.; Oliveira, D.; Martins, R.; Moreira, F.T.C.; Sales, M.G.F.; Fortunato, E. Paper-based platform with an in situ molecularly imprinted polymer for β-amyloid. ACS Omega 2020, 5, 12057–12066. [Google Scholar] [CrossRef]
- Karki, H.P.; Jang, Y.; Jung, J.; Oh, J. Advances in the development paradigm of biosample-based biosensors for early ultrasensitive detection of Alzheimer’s disease. J. Nanobiotechnol. 2021, 19, 72. [Google Scholar] [CrossRef]
- Parihar, A.; Gaur, K.; Khan, R. Rapid diagnostic assays for the detection of Alzheimer’s and Parkinson’s diseases. In Smart Diagnostics for Neurodegenerative Disorders: Neuro-Sensors; Elsevier: Amsterdam, The Netherlands, 2023; pp. 221–250. [Google Scholar] [CrossRef]
- Linnes, J.C.; Klapperich, C.M. Minimally instrumented paper-based molecular diagnostic for sexually transmitted infections. In Proceedings of the 18th International Conference on Miniaturized Systems for Chemistry and Life Sciences, MicroTAS 2014, San Antonio, TX, USA, 26–30 October 2014; pp. 1589–1591. [Google Scholar]
- Chiriacò, M.S.; Primiceri, E.; De Feo, F.; Montanaro, A.; Monteduro, A.G.; Tinelli, A.; Megha, M.; Carati, D.; Maruccio, G. Simultaneous detection of multiple lower genital tract pathogens by an impedimetric immunochip. Biosens. Bioelectron. 2016, 79, 9–14. [Google Scholar] [CrossRef]
- Rozand, C. Paper-based analytical devices for point-of-care infectious disease testing. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Kumalasari, M.R.; Alfanaar, R.; Andreani, A.S. Gold nanoparticles (AuNPs): A versatile material for biosensor application. Talanta Open 2024, 9, 100327. [Google Scholar] [CrossRef]
- Bahl, S.; Bagha, A.K.; Rab, S.; Javaid, M.; Haleem, A.; Singh, R.P. Advancements in biosensor technologies for medical field and COVID-19 pandemic. J. Ind. Integr. Manag. 2021, 6, 175–191. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Padhye, R.; Wang, X.; Houshyar, S. Advances in nanoparticle-enhanced paper sensor for detecting toxic metals in water. Talanta 2025, 293, 128146. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Hu, C.; Zhang, H.; Geng, S. Recent developments in paper-based sensors with instrument-free signal readout technologies (2020–2023). Biosensors 2024, 14, 36. [Google Scholar] [CrossRef]
- Sharma, A.; Kashyap, B.K.; Puranik, N. State-of-the-art of paper-based technology and challenges in its commercialization. In Paper-Based Diagnostic Devices for Infectious Diseases; IOP Publishing: Bristol, UK, 2023; pp. 11-1–11-15. [Google Scholar] [CrossRef]
- Adil, O.; Shamsi, M.H. Transformative biomedical devices to overcome biomatrix effects. Biosens. Bioelectron. 2025, 279, 117373. [Google Scholar] [CrossRef] [PubMed]
- Ratajczak, K.; Stobiecka, M. High-performance modified cellulose paper-based biosensors for medical diagnostics and early cancer screening: A concise review. Carbohydr. Polym. 2020, 229, 115463. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yang, X.; Wang, B.; Wang, L. Design and applications of fluorogenic nucleic acid-based paper biosensors. Prog. Chem. 2021, 33, 2309–2315. [Google Scholar] [CrossRef]
- Sinha, A.; Basu, M.; Chandna, P. Paper-based microfluidics: A forecast toward the most affordable and rapid point-of-care devices. Prog. Mol. Biol. Transl. Sci. 2022, 186, 109–158. [Google Scholar] [CrossRef]
- Hosain, M.N.; Kwak, Y.-S.; Lee, J.; Choi, H.; Park, J.; Kim, J. IoT-enabled biosensors for real-time monitoring and early detection of chronic diseases. Phys. Act. Nutr. 2024, 28, 60–69. [Google Scholar] [CrossRef]
- Ivanišević, I.; Milardović, S.; Kassal, P. Recent advances in (bio)chemical sensors for food safety and quality based on silver nanomaterials. Food Technol. Biotechnol. 2021, 59, 216–237. [Google Scholar] [CrossRef] [PubMed]
- Ukirade, N.; Kolhe, P. Recent advancement in electrochemical biosensor based on redox enzyme composite modified electrode for the detection of biomolecules. Suranaree J. Sci. Technol. 2024, 31, 030215. [Google Scholar] [CrossRef]
| Fabrication Technique | Description | Advantages | Limitations | Applications |
|---|---|---|---|---|
| Wax and Screen Printing [29]. | Printing methods to pattern hydrophobic and conductive regions on paper substrates. | Simple, cost-effective, eco-friendly, adaptable to origami structures. | Limited design complexity; may require post-processing. | Multi-analyte detection, origami devices, point-of-care diagnostics. |
| Robotic Printing and Micromanipulation [30]. | Uses robotic systems to integrate semiconductor microtubes into paper devices. | High precision, automated, versatile for electronics and biosensors. | Complex setup; requires advanced control systems. | Field-effect transistor (FET) biosensors, microelectronics. |
| Movable Type Bioelectronics Printing [31]. | Transfers bioelectronic materials via modular, pre-fabricated molds. | Flexible, low-cost, direct transfer of bioactive compounds. | Limited throughput; best for small-scale prototyping. | Continuous glucose and lactate monitoring. |
| Pen-Writing Technique [32]. | Employs rollerball pens with reagent inks to directly draw sensing patterns. | Affordable, customizable, barrier-free fabrication. | Limited to simple, manually drawn structures. | On-site bioassays, multi-analyte diagnostics. |
| Laser-Induced Graphene (LIG) [33]. | Generates conductive graphene directly on paper via laser irradiation. | High conductivity, flexible, disposable. | Requires specialized laser equipment; two-step processing. | Glucose biosensors, enzymatic electrochemical devices. |
| Aerosol-Assisted PECVD [34]. | Deposits bioreactive and biorepellent layers using plasma-enhanced chemical vapor deposition. | Fast, stable, adherent, reproducible coatings. | Expensive and equipment-intensive. | DNA detection, nucleic acid biosensors. |
| Roll-to-Roll Processing [35]. | Employs continuous printing and deposition methods for large-scale production. | Scalable, high-speed, low-cost manufacturing. | Requires integration of multiple techniques; equipment intensive. | Mass production of fully printed biosensors. |
| Pencil Drawing Method [11,36]. | Uses graphite pencils to create conductive traces on paper. | Extremely low-cost, reproducible, simple fabrication. | Restricted to graphite-based electrodes. | Electrochemical detection of E. coli and pathogens. |
| Bottom-Up Wax-Patterned Microchip Printing [12,37]. | Builds translucent microfluidic arrays from cellulose fibers with wettability patterning. | High resolution, rapid prototyping, transparent structures. | Paper porosity limits resolution; requires careful optimization. | Glucose monitoring, flexible wearable biosensors. |
| Year | Study Title | Study Person(s) | Findings |
|---|---|---|---|
| 2023 | “Printable biosensors towards next-generation point-of-care testing: Paper substrate as an example” | Liu, Y., Lu, S., Zhang, Z., & Liu, G. | Showed printing technologies (wax, screen, photolithography, inkjet, and laser) improve precision, efficiency, and scalability of paper biosensors [9]. |
| 2022 | “Nanomaterials and paper-based electrochemical devices: Emerging strategies for detection of biomarkers” | Caratelli, V., Di Meo, E., Colozza, N., & Arduini, F. | Integration of nanomaterials enhanced sensitivity, selectivity, and sustainability in biomarker detection [61]. |
| 2024 | “Gold nanoparticles (AuNPs): A versatile material for biosensor application” | Kumalasari, M.R., Alfanaar, R., & Andreani, A.S. | Demonstrated that AuNPs increase electroactive surface area and detection accuracy [127]. |
| 2024 | “Paper-based DNA biosensor for rapid and selective detection of miR-21” | Hunt, A., Torati, S.R., & Slaughter, G. | Developed inkjet-printed gold biosensor for rapid miR-21 cancer biomarker detection [73]. |
| 2021 | “Advancements in biosensor technologies for medical field and COVID-19 pandemic” | Bahl, S., Bagha, A.K., Rab, S., & Singh, R.P. | Showed rapid electrochemical biosensors for COVID-19 detection and medical diagnostics [128]. |
| 2025 | “Advances in nanoparticle-enhanced paper sensor for detecting toxic metals in water” | Hosseini, M.S., Padhye, R., Wang, X., & Houshyar, S. | Nanoparticle functionalization enabled sensitive on-site detection of toxic metals in water [129]. |
| 2024 | “MOF-mediated paper-based (bio)sensors for detecting food and environmental pollutants” | Huang, D., Ma, H., Wang, J., & Li, R. | Demonstrated MOFs integrated with paper substrates for rapid and accurate detection of food/environmental contaminants [16]. |
| 2024 | “Recent developments in paper-based sensors with instrument-free signal readout technologies (2020–2023)” | Yang, D., Hu, C., Zhang, H., & Geng, S. | Innovated distance-based, counting-based, and text-based readout methods for instrument-free detection [130]. |
| 2023 | “State-of-the-art of paper-based technology and challenges in its commercialization” | Sharma, A., Kashyap, B.K., & Puranik, N. | Highlighted commercialization barriers such as large-scale reproducibility and clinical validation challenges [131]. |
| 2025 | “Transformative biomedical devices to overcome biomatrix effects” | Adil, O., & Shamsi, M.H. | Addressed biomatrix interference in clinical samples with improved biosensor designs [132]. |
| 2020 | “High-performance modified cellulose paper-based biosensors for medical diagnostics and early cancer screening: A concise review” | Ratajczak, K., & Stobiecka, M. | Reviewed modified cellulose paper substrates enhancing performance in medical diagnostics and early cancer detection [133]. |
| 2023 | “Paper-based biosensors: Overview from past to future” | Shrikrishna, N.S., Sharma, R., & Gandhi, S. | Provided a comprehensive overview of paper-based biosensors, highlighting trends, applications, and future directions [2]. |
| 2021 | “Design and applications of fluorogenic nucleic acid-based paper biosensors” | Yang, S., Yang, X., Wang, B., & Wang, L. | Developed fluorescent biosensors with functional nucleic acids for sensitive and real-time detection [134]. |
| 2023 | “Paper-based electrochemical biosensors for the diagnosis of viral diseases” | Ataide, V.N., Pradela-Filho, L.A., Ameku, W.A., & Angnes, L. | Presented electrochemical biosensors for diagnosing viral diseases such as COVID-19, dengue, and Zika [4]. |
| 2022 | “Paper-based microfluidics: A forecast toward the most affordable and rapid point-of-care devices” | Sinha, A., Basu, M., & Chandna, P. | Predicted future trends of paper-based microfluidics for rapid and affordable point-of-care diagnostics [135]. |
| 2024 | “IoT-enabled biosensors for real-time monitoring and early detection of chronic diseases” | Hosain, M.N., Kwak, Y.-S., Lee, J., & Kim, J. | Developed IoT-enabled biosensors for real-time monitoring and early diagnosis of chronic diseases [136]. |
| 2021 | “Recent advances in (bio)chemical sensors for food safety and quality based on silver nanomaterials” | Ivanišević, I., Milardović, S., & Kassal, P. | Reviewed silver nanomaterial-based sensors for food safety and quality monitoring [137]. |
| 2024 | “Emerging diagnostic methods using paper-based electrochemical biosensors” | Karuppannan, P.G., Sudha, D., Banupriya, K., Arumugam, R. | Highlighted emerging diagnostic methods using paper-based electrochemical biosensors [1]. |
| 2020 | “Cytokine and cancer biomarkers detection: The dawn of electrochemical paper-based biosensor” | Loo, S.W., Pui, T.-S. | Showed sensitive detection of cytokines and cancer biomarkers using electrochemical paper-based biosensors [3]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Das, A.; Borthakur, P.P.; Das, D.; Sahariah, J.J.; Kalita, P.; Pathak, K. Emerging Trends in Paper-Based Electrochemical Biosensors for Healthcare Applications. Eng. Proc. 2025, 106, 8. https://doi.org/10.3390/engproc2025106008
Das A, Borthakur PP, Das D, Sahariah JJ, Kalita P, Pathak K. Emerging Trends in Paper-Based Electrochemical Biosensors for Healthcare Applications. Engineering Proceedings. 2025; 106(1):8. https://doi.org/10.3390/engproc2025106008
Chicago/Turabian StyleDas, Aparoop, Partha Protim Borthakur, Dibyajyoti Das, Jon Jyoti Sahariah, Parimita Kalita, and Kalyani Pathak. 2025. "Emerging Trends in Paper-Based Electrochemical Biosensors for Healthcare Applications" Engineering Proceedings 106, no. 1: 8. https://doi.org/10.3390/engproc2025106008
APA StyleDas, A., Borthakur, P. P., Das, D., Sahariah, J. J., Kalita, P., & Pathak, K. (2025). Emerging Trends in Paper-Based Electrochemical Biosensors for Healthcare Applications. Engineering Proceedings, 106(1), 8. https://doi.org/10.3390/engproc2025106008







