Wearable Biosensors for Glucose Monitoring in Sweat: A Patent Analysis †
Abstract
1. Introduction
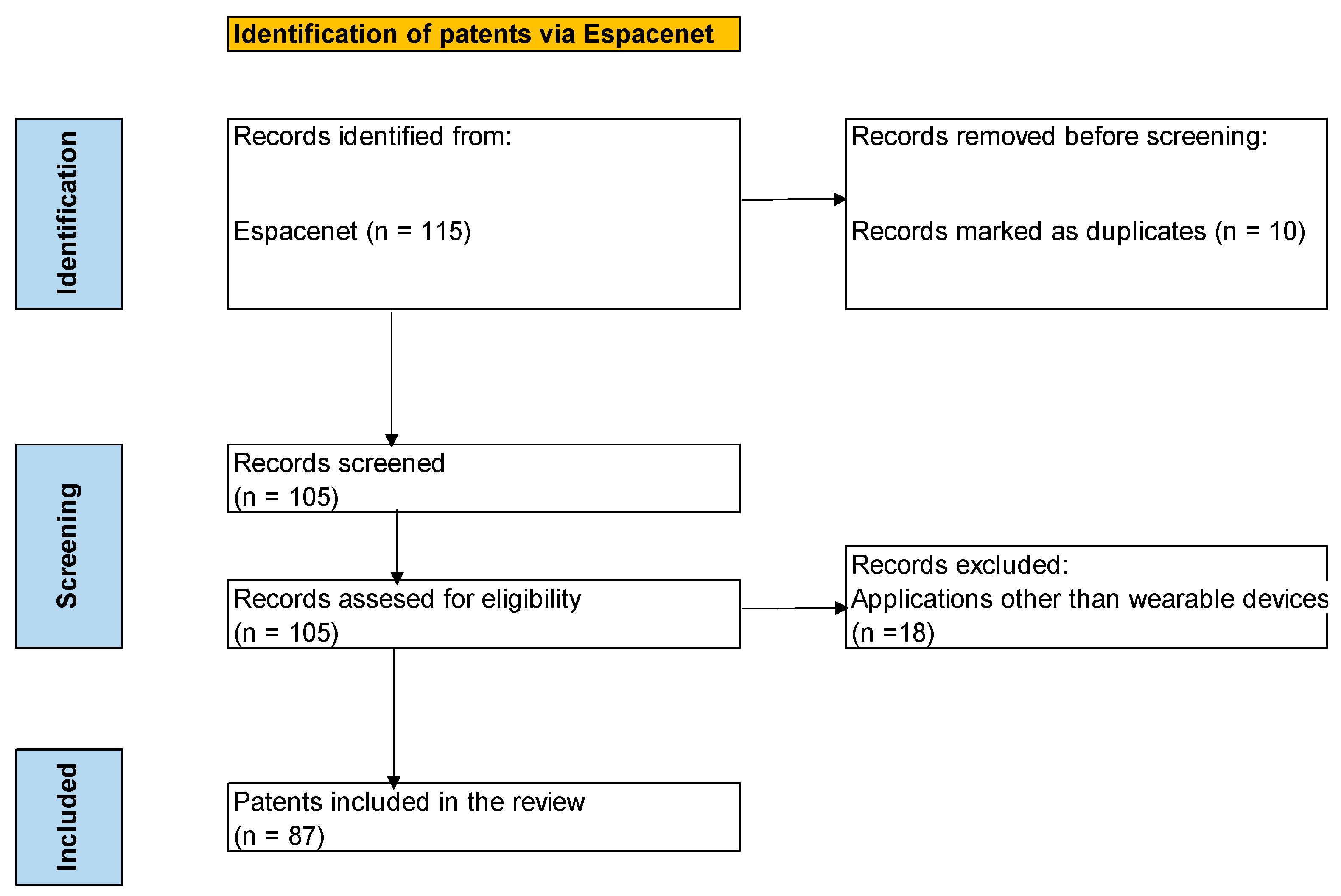
2. Materials and Methods
- Patent applications or those granted using sweat as the biological fluid dedicated to the measurement of glucose levels.
- Patent applications or those granted filed in a 20-year period, starting from January 2005 and before 16 December 2024.
- Patents or patent applications pertaining to a wearable device designed for the continuous monitoring of glucose levels in sweat.
3. Results and Discussion
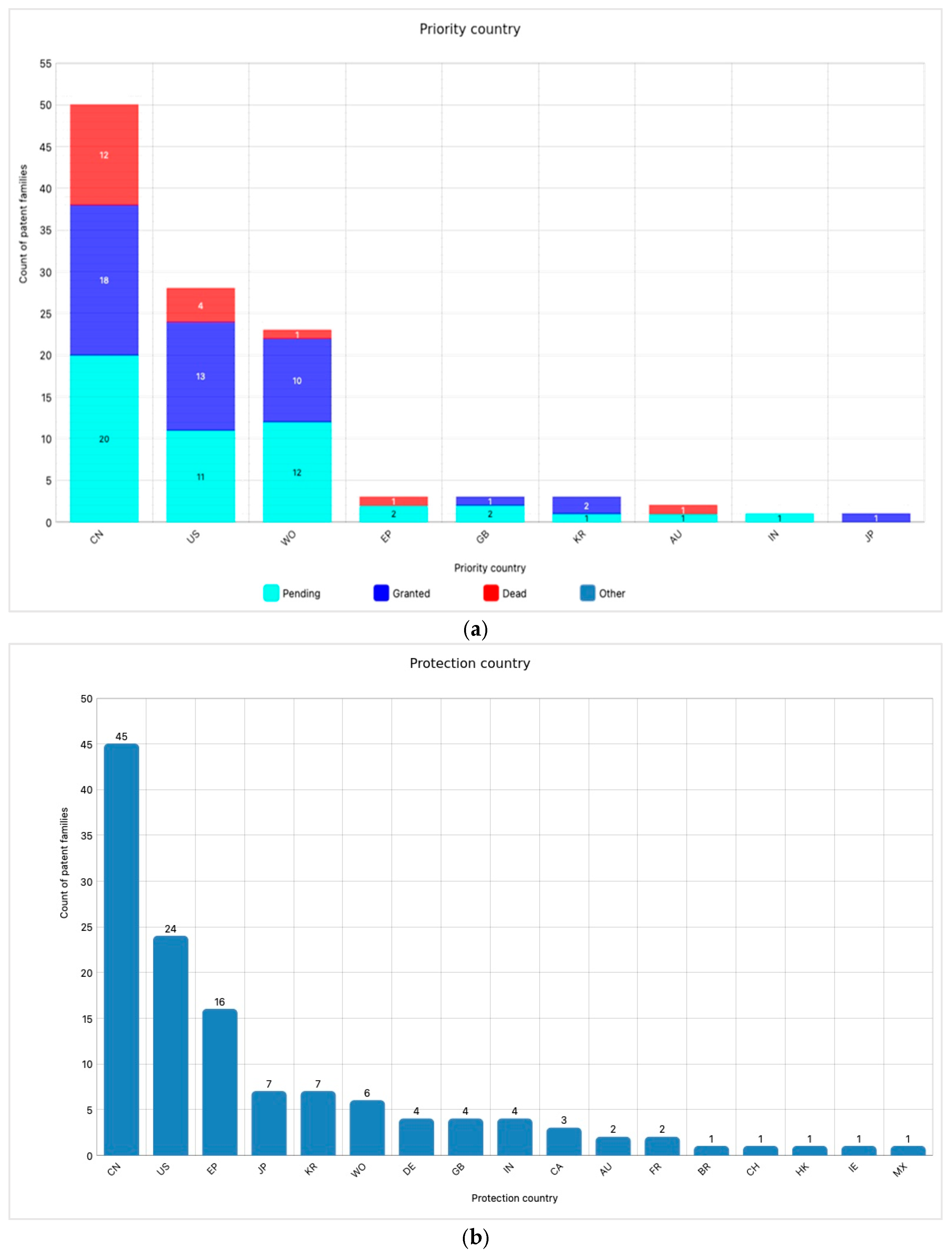
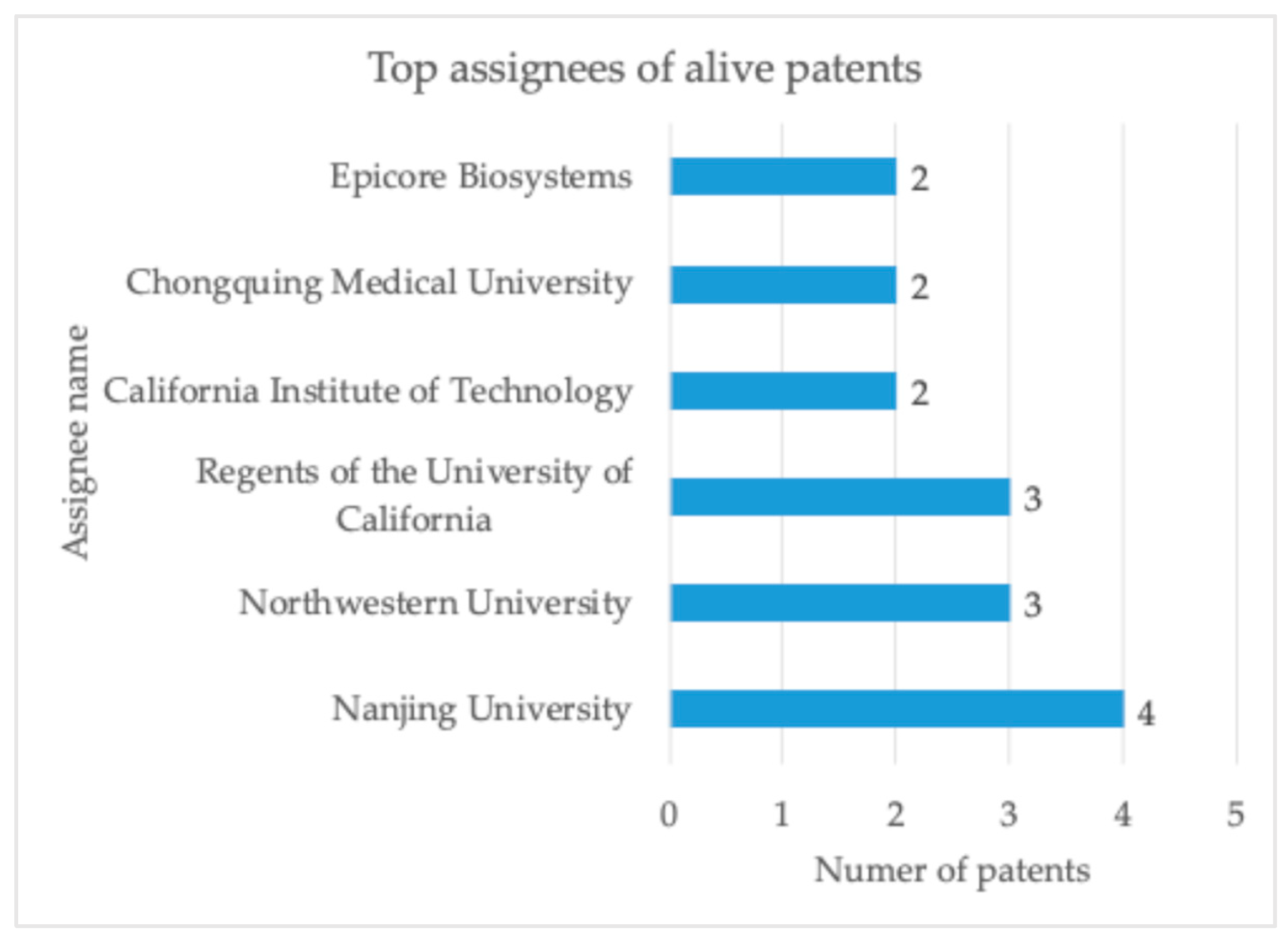
3.1. Academic and Industrial Research
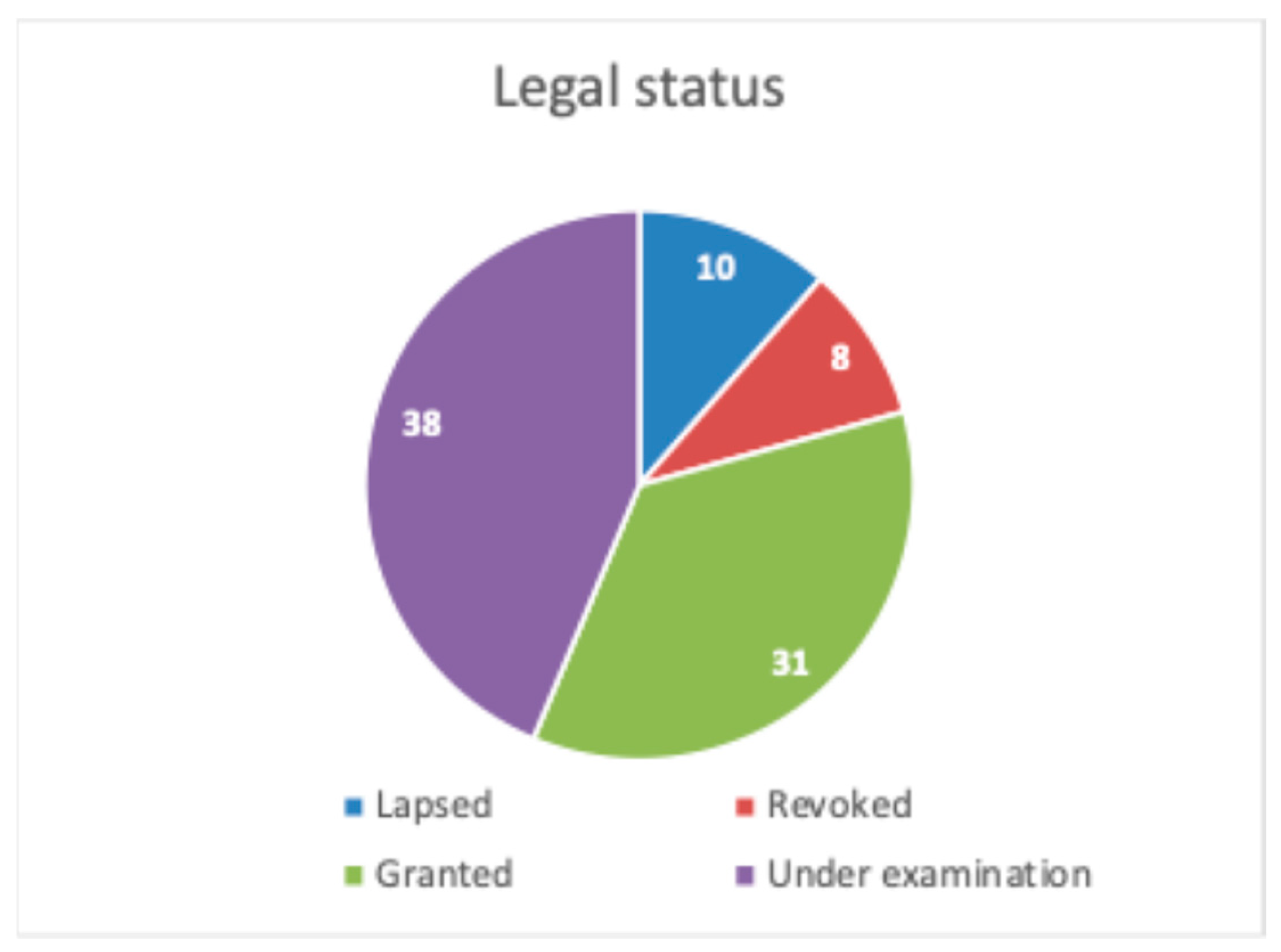
3.2. IP Exploitation and Patented Products on the Market
- The minor reliability of the technologies based on sweat with respect to the blood sampling techniques: Even if the performances are quite good, the lack of selectivity in sweat is still shown and the sensitivity is still to be improved considering low glucose concentration, ranging from 0.02 to 0.6 mM [4], to reach the same accuracy.
- The occurrence of new long-term sensors deployed in the market: Continuous Glucose Monitoring through a small patch able to measure glucose levels in the interstitial fluid for up to 14 days is today the gold-standard reference of the technology. With this system, blood sampling is no longer required, and the mobile app can directly read the glucose levels with a simple gesture. Its reliability and simplicity of use are far from being achieved by sweat analysis devices. These systems were first introduced to the market in fall 2014. Its clinical adoption was gradual but rapidly grew for its revolution in the users’ lifestyles. With the new CGM sensor generations released to the market (today we are at the third generation), after a few years, they have become the reference devices; this is the most probable reason for the decrease in patent activity for sweat sensors after 2021.
- Medical resilience is another barrier: Doctors are quite stable in their device adoption because they have also developed efficient diabetes management strategies for categories of patients (with the possibility of customization programs) with large numbers of subjects. The availability of a new technology implies a long period of knowledge acquisition and testing with several patients before reaching the same level of awareness and efficacy in the development of customizable clinical programs.
- The regulatory constraints are the final remark to be considered: The new MDR 645/2017 regulation—entered into force in 2021 in Europe—requires more investments and activity to fulfill its obligations.
3.3. Patent Strategies and Freedom-to-Operate Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| FTO | Freedom-to-Operate search |
| TAC | Title Abstract Claim |
| CPC | Cooperative Patent Classification |
| IPC | International Patent Classification |
| PCT | Patent Cooperation Treaty |
| CNTs | Carbon nanotubes |
| GOx | Glucose Oxidase |
| FAD | Flavin adenine dinucleotide |
| rGO | Reduced Graphene Oxide |
| MOF | Metal–Organic Framework |
| GDH | Glucose dehydrogenase |
| LIG | Laser-induced graphene |
| LOx | Lactate oxidase |
Appendix A
References
- Ameen, S.S.M.; Omer, K.M.; Mansour, F.R.; Bedair, A.; Hamed, M. Non-invasive wearable electrochemical sensors for continuous glucose monitoring. Electrochem. Commun. 2025, 173, 107894. [Google Scholar] [CrossRef]
- Zafar, H.; Channa, A.; Jeoti, V.; Stojanovic, G.M. Comprehensive Review on Wearable Sweat-Glucose Sensors for Continuous Glucose Monitoring. Sensors 2022, 22, 638. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chang, S.J.; Chen, C.-J.; Liu, J.-T. Non-Invasive Blood Glucose Monitoring Technology: A Review. Sensors 2020, 20, 6925. [Google Scholar] [CrossRef] [PubMed]
- Moyer, J.; Wilson, D.; Finkelshtein, I.; Wong, B.; Potts, R. Correlation Between Sweat Glucose and Blood Glucose in Subjects with Diabetes. Diabetes Technol. Ther. 2012, 14, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Espacenet Patent Search. Available online: https://worldwide.espacenet.com (accessed on 16 December 2024).
- Orbit Patent Intelligence. Available online: https://www.orbit.com (accessed on 16 December 2024).
- WIPO IPC Publication. Available online: https://ipcpub.wipo.int (accessed on 1 June 2025).
- CPC Scheme and Definitions. Available online: https://www.cooperativepatentclassification.org/cpcSchemeAndDefinitions/table (accessed on 1 June 2025).
- Sunstrum, F.N.; Khan, J.U.; Li, N.-W.; Welch, A.W. Wearable textile sensors for continuous glucose monitoring. Biosens. Bioelectron. 2025, 273, 117133. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Li, X.; Zhou, L.; Su, B. An Overview of Wearable and Implantable Electrochemical Glucose Sensors. Electroanalysis 2022, 34, 237–245. [Google Scholar] [CrossRef]
- Huang, Q.; Chen, J.; Zhao, Y.; Huang, J.; Liu, H. Advancements in electrochemical glucose sensors. Talanta 2025, 281, 126897. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yao, C.; Huang, S.; Zheng, S.; Liu, Z.; Liu, J.; Wang, J.; Chen, H.-J.; Xie, X. Technological Advances of Wearable Device for Continuous Monitoring of In Vivo Glucose. ACS Sens. 2024, 9, 1065–1088. [Google Scholar] [CrossRef] [PubMed]
- Saha, T.; Del Caño, R.; Mahato, K.; Dela Paz, E.; Chen, C.; Ding, S.; Yin, L.; Wang, J. Wearable Electrochemical Glucose Sensors in Diabetes Management: A Comprehensive Review. Chem. Rev. 2023, 123, 7854–7889. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D.-H. Enzyme-Based Glucose Sensor: From Invasive to Wearable Device. Adv. Healthc. Mater. 2018, 7, 1701150. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, W.; Wu, J.; Li, H.; Peng, H.; Zhang, J.; Ding, K.; Wang, X.; Hou, C.; Zhang, H.; et al. Smart Wearable Sensor Fuels Noninvasive Body Fluid Analysis. ACS Appl. Mater. Interfaces 2025, 17, 13279–13301. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lin, X.; Li, Z.; Zhang, X.; Chen, Y.; Zheng, Z.; Liu, Y.; Xia, Y.; Mou, L. Wearable Sweat Detection Device. Chinese Patent Application No. CN118830835A. Available online: https://worldwide.espacenet.com/patent/search/family/093142176/publication/CN118830835A?q=CN118830835A (accessed on 1 June 2025).
- Liu, H.; Liu, H. Blood Glucose Monitoring System. Chinese Patent No. CN106923842B. Available online: https://worldwide.espacenet.com/patent/search/family/059432939/publication/CN106923842B?q=CN106923842B (accessed on 2 June 2025).
- Cai, K.; Wang, L. Preparation Method of Flexible Wearable Sensor for Real-Time Monitoring of Glucose in Sweat. Chinese Patent Application No. CN118191059A. Available online: https://worldwide.espacenet.com/patent/search/family/091407985/publication/CN118191059A?q=CN118191059A (accessed on 2 June 2025).
- Lyu, G.; Ren, G. Preparation Method and Application of All-Carbon Organic Electrochemical Transistor Based on Laser-Induced Graphene. Chinese Patent No. CN115950942B. Available online: https://worldwide.espacenet.com/patent/search/family/087285522/publication/CN115950942B?q=CN115950942 (accessed on 2 June 2025).
- Gu, C.; Liang, W.; Zhang, L.; Ge, P.; Li, F. Flexible Wearable Self-Powered Sensor System for In-Situ Detection of Glucose in Sweat and Application of Flexible Wearable Self-Powered Sensor System. Chinese Patent Application No. CN117269261A. Available online: https://worldwide.espacenet.com/patent/search/family/089220640/publication/CN117269261A?q=CN117269261A (accessed on 2 June 2025).
- Jiang, X.; Mou, L. Glucose Electrode, Micro-Fluidic Chip, Micro-Fluidic Passive Sweat Patch, and Preparation Method and Application of Micro-Fluidic Passive Sweat Patch. Chinese Patent Application No. CN112697857A. Available online: https://worldwide.espacenet.com/patent/search/family/075506318/publication/CN112697857A?q=CN112697857A (accessed on 2 June 2025).
- Wang, J.; Bandodkar, A.J.; Mercier, P. Non-Invasive and Wearable Chemical Sensors and Biosensors. US Patent No. US10722160B2. Available online: https://worldwide.espacenet.com/patent/search/family/056092499/publication/US10722160B2?q=US10722160B2 (accessed on 2 June 2025).
- Javey, A.; Gao, W.; Davis, R.W.; Emaminejad, S. Wearable Sensor Array for In-Situ Body Fluid Analysis. US Patent Application No. US20180263539A1; (Rejected in 2020), Available online: https://worldwide.espacenet.com/patent/search/family/058427319/publication/US2018263539A1?q=US20180263539A1 (accessed on 2 June 2025).
- Yi, Y.; Chen, Y.; Zang, G.; Sun, Y.; Wen, Z.; Zhang, Y. Compound for Continuously Detecting Glucose Content in Sweat, Sensor and Application. Chinese Patent Application No. CN118237080A. Available online: https://worldwide.espacenet.com/patent/search/family/091555891/publication/CN118237080A?q=pn%3DCN118237080A (accessed on 2 June 2025).
- Chen, Y.; Sun, Y.; Li, Y.; Peng, X.; He, Y.; Hou, Y.; Fan, J.; Zang, G.; Zhang, Y. A wearable non-enzymatic sensor for continuous monitoring of glucose in human sweat. Talanta 2024, 278, 126499. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Dan, J.; Chen, J. Glucose oxidase nanocapsule sensor and preparation and application thereof. Chinese Patent No. CN111624244 (B). Available online: https://worldwide.espacenet.com/publicationDetails/biblio?CC=CN&NR=111624244B&KC=B&FT=D&ND=4&date=20211221&DB=&locale=en_EP (accessed on 8 August 2025).
- Nidarsha, K.M.; Rangika, P.L.H.; Madhu, H.G.H.C.; Ruvini, R.H.H.A.M.; Madushika, P.K. Nonenzymatic electrochemical sensors. PCT patent publication No. WO2021148952 (A1). Available online: https://worldwide.espacenet.com/publicationDetails/biblio?II=0&ND=3&adjacent=true&locale=en_EP&FT=D&date=20210729&CC=WO&NR=2021148952A1&KC=A1 (accessed on 8 August 2025).
- GX Sweat Patch. Available online: https://www.epicorebiosystems.com/our-solutions/gx-sweat-patch (accessed on 2 June 2025).
- OnaVital. Available online: https://onalabs.com/en/onavital/ (accessed on 2 June 2025).
- Limina Muscular Biosensor. Available online: https://www.liminafitness.com/the-science (accessed on 2 June 2025).
- NIX Hydration Biosensor. Available online: https://nixbiosensors.com (accessed on 2 June 2025).
- HDROP Hydration Wearable Monitor Sensor. Available online: https://hdroptech.com/ (accessed on 8 August 2025).
- Yehezkeli, O.; Cohen, R.; Cohen, Y.; Mukah, D. Oxygen Insentive Amperometric Biosensors. PCT application No. WO2022054044A1. Available online: https://worldwide.espacenet.com/patent/search/family/077774957/publication/WO2022054044A1?q=pn%3DWO2022054044A1 (accessed on 2 June 2025).
- Su, T.; Xia, Y.; Zhang, S.; Shu, Y. Preparation Method of Flexible Wearable Sweat Glucose Electrochemical Sensor. Chinese Patent Application No. CN114674885A; (Now Rejected), Available online: https://worldwide.espacenet.com/patent/search/family/082074779/publication/CN114674885A?q=pn%3DCN114674885A (accessed on 2 June 2025).
- Shu, Y.; Su, T.; Shang, Z.; Xu, Q.; Hu, X. Highly Stretchable Wearable Electrochemical Sensor Based on Ni-Co MOF Nanosheet-Decorated Ag/rGO/PU Fiber for Continuous Sweat Glucose Detection. Anal. Chem. 2021, 93, 16222–16230. [Google Scholar] [CrossRef] [PubMed]
- Ren, G.; Fan, H.; Zhang, L.; He, S.; Zhu, C.; Gao, K.; Zhang, Y.; Wang, J.; Kang, X.; Gong, Z.; et al. A laser-induced graphene-based flexible and all-carbon organic electrochemical transistor. J. Mater. Chem. C 2023, 11, 4916–4928. [Google Scholar] [CrossRef]


| CPC Codes | % | Definition |
|---|---|---|
| A61B5/145 | 57.47% | Measuring the concentration of an analyte in a body fluid |
| A61B5/14532 | 47.13% | For measuring glucose |
| A61B5/14517 | 36.78% | For sweat |
| G01N27/327 | 33.33% | Biochemical electrodes |
| A61B5/14546 | 26.44% | For measuring analytes not otherwise provided |
| A61B5/00 | 22.99% | Measuring for diagnostic purposes |
| A61B5/1477 | 22.99% | Non-invasive |
| A61B5/6833 | 18.39% | Adhesive patches |
| A61B5/1486 | 17.24% | Using enzyme electrodes (e.g., with immobilized oxidase) |
| A61B5/01 | 14.94% | Measuring temperature of body parts |
| A61B5/681 | 13.79% | Wristwatch-type devices |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbieri, M.; Andreoni, G. Wearable Biosensors for Glucose Monitoring in Sweat: A Patent Analysis. Eng. Proc. 2025, 106, 1. https://doi.org/10.3390/engproc2025106001
Barbieri M, Andreoni G. Wearable Biosensors for Glucose Monitoring in Sweat: A Patent Analysis. Engineering Proceedings. 2025; 106(1):1. https://doi.org/10.3390/engproc2025106001
Chicago/Turabian StyleBarbieri, Massimo, and Giuseppe Andreoni. 2025. "Wearable Biosensors for Glucose Monitoring in Sweat: A Patent Analysis" Engineering Proceedings 106, no. 1: 1. https://doi.org/10.3390/engproc2025106001
APA StyleBarbieri, M., & Andreoni, G. (2025). Wearable Biosensors for Glucose Monitoring in Sweat: A Patent Analysis. Engineering Proceedings, 106(1), 1. https://doi.org/10.3390/engproc2025106001







