Development of Biochar-Based Sustainable Corrosion-Resistant Coating †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Biochar
2.3. Synthesis of Coating Material
2.4. Preparation of Coating Panels
3. Result and Discussion
3.1. Yield of Biochar
3.2. Fourier Transform Infrared Spectroscopy (FTIR)
3.3. Field Emission Scanning Electron Microscopy with EDX (FESEM-EDX)
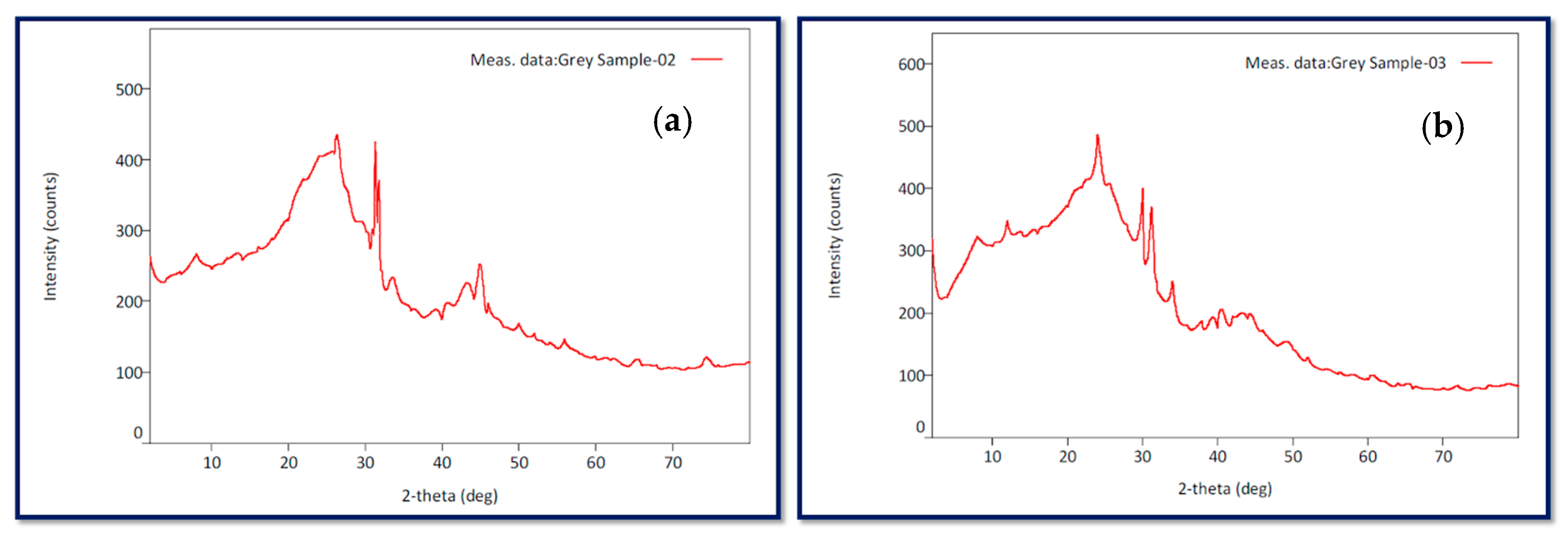
3.4. X-Ray Diffraction (XRD)
3.5. Study on the Effect of Biochar on Coating Performance
3.5.1. Gloss of the Coating
3.5.2. Mechanical Properties of Coating
3.5.3. Chemical Properties of Coating
3.5.4. Effect of Biochar on Water Contact Angle of Coating
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FTIR | Fourier Transform Infrared Spectroscopy |
| FESEM-EDX | Field Emission Scanning Electron Microscopy with EDX |
| XRD | X-ray Diffraction |
| VOC | Volatile Organic Compounds |
| ASTM | American Standard for Testing and Materials |
| WCA | Water Contact Angle |
References
- Fotovvati, B.; Namdari, N.; Dehghanghadikolaei, A. On coating techniques for surface protection: A review. J. Manuf. Mater. Process. 2019, 3, 28. [Google Scholar] [CrossRef]
- Mo, Z.; Lu, S.; Shao, M. Volatile organic compound (VOC) emissions and health risk assessment in paint and coatings industry in the Yangtze River Delta, China. Environ. Pollut. 2021, 269, 115740. [Google Scholar] [CrossRef]
- Zhou, X.; Li, Y.; Fang, C.; Li, S.; Cheng, Y.; Lei, W.; Meng, X. Recent Advances in Synthesis of Waterborne Polyurethane and Their Application in Water-based Ink: A Review. J. Mater Sci. Technol. 2015, 31, 708–722. [Google Scholar] [CrossRef]
- Ahmad, M.; Rajapaksha, A.U.; Lim, J.E.; Zhang, M.; Bolan, N.; Mohan, D.; Vithanage, M.; Lee, S.S.; Ok, Y.S. Biochar as a sorbent for contaminant management in soil and water: A review. Chemosphere 2014, 99, 19–33. [Google Scholar] [CrossRef]
- Laird, D.A.; Brown, R.C.; Amonette, J.E.; Lehmann, J. Review of the pyrolysis platform for coproducing bio-oil and biochar. Biofuels Bioprod. Biorefining 2009, 3, 547–562. [Google Scholar] [CrossRef]
- Guo, F.; Bao, L.; Wang, H.; Larson, S.L.; Ballard, J.H.; Knotek-Smith, H.M.; Zhang, Q.; Su, Y.; Wang, X.; Han, F. A simple method for the synthesis of biochar nanodots using hydrothermal reactor. MethodsX 2020, 7, 101022. [Google Scholar] [CrossRef] [PubMed]
- Lian, F.; Xing, B. Black Carbon (Biochar) in Water/Soil Environments: Molecular Structure, Sorption, Stability, and Potential Risk. Am. Chem. Soc. 2017, 51, 13517–13532. [Google Scholar] [CrossRef] [PubMed]
- Amin, F.R.; Huang, Y.; He, Y.; Zhang, R.; Liu, G.; Chen, C. Biochar applications and modern techniques for characterization. Clean Technol. Environ. Policy 2016, 18, 1457–1473. [Google Scholar] [CrossRef]
- Mukherjee, A.; Patra, B.R.; Podder, J.; Dalai, A.K. Synthesis of Biochar from Lignocellulosic Biomass for Diverse Industrial Applications and Energy Harvesting: Effects of Pyrolysis Conditions on the Physicochemical Properties of Biochar. Front. Mater. 2022, 9, 870184. [Google Scholar] [CrossRef]
- Leng, L.; Xiong, Q.; Yang, L.; Li, H.; Zhou, Y.; Zhang, W.; Jiang, S.; Li, H.; Huang, H. An overview on engineering the surface area and porosity of biochar. Sci. Total Environ. 2021, 763, 144204. [Google Scholar] [CrossRef]
- Visser, E.D.; Seroka, N.S.; Khotseng, L. Recent Advances in Biochar: Synthesis Techniques, Properties, Applications, and Hydrogen Production. Processes 2024, 12, 1111. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Adel, O.; Khamis, E. Fabrication of biochar-based superhydrophobic coating on steel substrate and its UV resistance, anti-scaling, and corrosion resistance performance. Sci. Rep. 2023, 13, 9453. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Y.; Sun, Y.; Feng, F.; Tagawa, K. Preparation of biochar-based photothermal superhydrophobic coating based on corn straw biogas residue and blade anti-icing performance by wind tunnel test. Renew Energy 2023, 210, 618–626. [Google Scholar] [CrossRef]
- Zhang, M.; Zhu, H.; Xi, B.; Tian, Y.; Sun, X.; Zhang, H.; Wu, B. Surface Hydrophobic Modification of Biochar by Silane Coupling Agent KH-570. Processes 2022, 10, 301. [Google Scholar] [CrossRef]
- Zhu, W.; Li, X.; Liu, X.; Bai, L.; Wang, X.; Li, A.; Han, Y.; Wei, C.; Dong, J.; Guo, Z.; et al. Enhancing the corrosion resistance of waterborne epoxy coatings with functionalized biochar. RSC Adv. 2024, 14, 39747–39758. [Google Scholar] [CrossRef]
- Lei, Y.; Hu, L.; Du, S.; Xu, D.; Yang, J. Fluorine-Free and Robust Photothermal Superhydrophobic Coating Based on Biochar for Anti-/De-Icing. Coatings 2024, 14, 838. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, G.; Ma, S.; Wang, H.; Bai, J. Enhancement of barrier and corrosion protection performance of epoxy coatings through adding eco-friendly lamellar biochar. Mater. Corros. 2022, 73, 720–732. [Google Scholar] [CrossRef]
- Li, Z.; Ravenni, G.; Bi, H.; Weinell, C.E.; Ulusoy, B.; Zhang, Y.; Dam-Johansen, K. Effects of biochar nanoparticles on anticorrosive performance of zinc-rich epoxy coatings. Prog. Org. Coat. 2021, 158, 106351. [Google Scholar] [CrossRef]
- Amirchand, K.D.; Kaur, K.; Singh, V. Biochar based self cleaning superhydrophobic surface with aqueous DESphobic properties. J. Mol. Liq. 2023, 380, 121736. [Google Scholar] [CrossRef]
- Nwajiaku, I.M.; Olanrewaju, J.S.; Sato, K.; Tokunari, T.; Kitano, S.; Masunaga, T. Change in nutrient composition of biochar from rice husk and sugarcane bagasse at varying pyrolytic temperatures. Int. J. Recycl. Org. Waste Agric. 2018, 7, 269–276. [Google Scholar] [CrossRef]
- Khare, P. A comprehensive evaluation of inherent properties and applications of nano-biochar prepared from different methods and feedstocks. J. Clean. Prod. 2021, 320, 128759. [Google Scholar] [CrossRef]
- Ramanayaka, S.; Vithanage, M.; Alessi, D.S.; Liu, W.J.; Jayasundera, A.C.A.; Ok, Y.S. Nanobiochar: Production, properties, and multifunctional applications. Environ. Sci. Nano 2020, 7, 3279–3302. [Google Scholar] [CrossRef]
- Jiang, M.; He, L.; Niazi, N.K.; Wang, H.; Gustave, W.; Vithanage, M.; Geng, K.; Shang, H.; Zhang, X.; Wang, Z. Nanobiochar for the remediation of contaminated soil and water: Challenges and opportunities. Biochar 2023, 5, 2. [Google Scholar] [CrossRef]




| Epoxy Resin % by Weight | SW800 % by Weight | SW1000 % by Weight | Solvent % by Weight | Total | |
|---|---|---|---|---|---|
| Batch-1 (Biochar-free coating) | 90 | - | - | 10 | 100 |
| Batch-2 | 90 | 0.9 | - | 9.1 | 100 |
| Batch-3 | 90 | 1.8 | - | 8.2 | 100 |
| Batch-4 | 90 | 2.7 | - | 7.3 | 100 |
| Batch-5 | 90 | 3.6 | - | 6.4 | 100 |
| Batch-6 | 90 | 4.5 | - | 5.5 | 100 |
| Batch-7 | 90 | - | 0.9 | 9.1 | 100 |
| Batch-8 | 90 | - | 1.8 | 8.2 | 100 |
| Batch-9 | 90 | - | 2.7 | 7.3 | 100 |
| Batch-10 | 90 | - | 3.6 | 6.4 | 100 |
| Batch-11 | 90 | - | 4.5 | 5.5 | 100 |
| Biochar Loading (%) | Batch SW800 (Gloss in GU) | SW1000 (Gloss in GU) |
|---|---|---|
| 1% | 79.2 | 81.4 |
| 2% | 75.6 | 77.7 |
| 3% | 74.0 | 75.4 |
| 4% | 73.8 | 74.6 |
| 5% | 72.5 | 73.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zade, G.; Kulkarni, M. Development of Biochar-Based Sustainable Corrosion-Resistant Coating. Eng. Proc. 2025, 105, 5. https://doi.org/10.3390/engproc2025105005
Zade G, Kulkarni M. Development of Biochar-Based Sustainable Corrosion-Resistant Coating. Engineering Proceedings. 2025; 105(1):5. https://doi.org/10.3390/engproc2025105005
Chicago/Turabian StyleZade, Ganesh, and Malhari Kulkarni. 2025. "Development of Biochar-Based Sustainable Corrosion-Resistant Coating" Engineering Proceedings 105, no. 1: 5. https://doi.org/10.3390/engproc2025105005
APA StyleZade, G., & Kulkarni, M. (2025). Development of Biochar-Based Sustainable Corrosion-Resistant Coating. Engineering Proceedings, 105(1), 5. https://doi.org/10.3390/engproc2025105005






