Investigation of Laser-Based Mo-Doped ZnO Nanoparticle Production and Photocatalysis Application †
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Linear Absorbance of Mo Nps, ZnO Nps, and Mo-Doped ZnO Nps
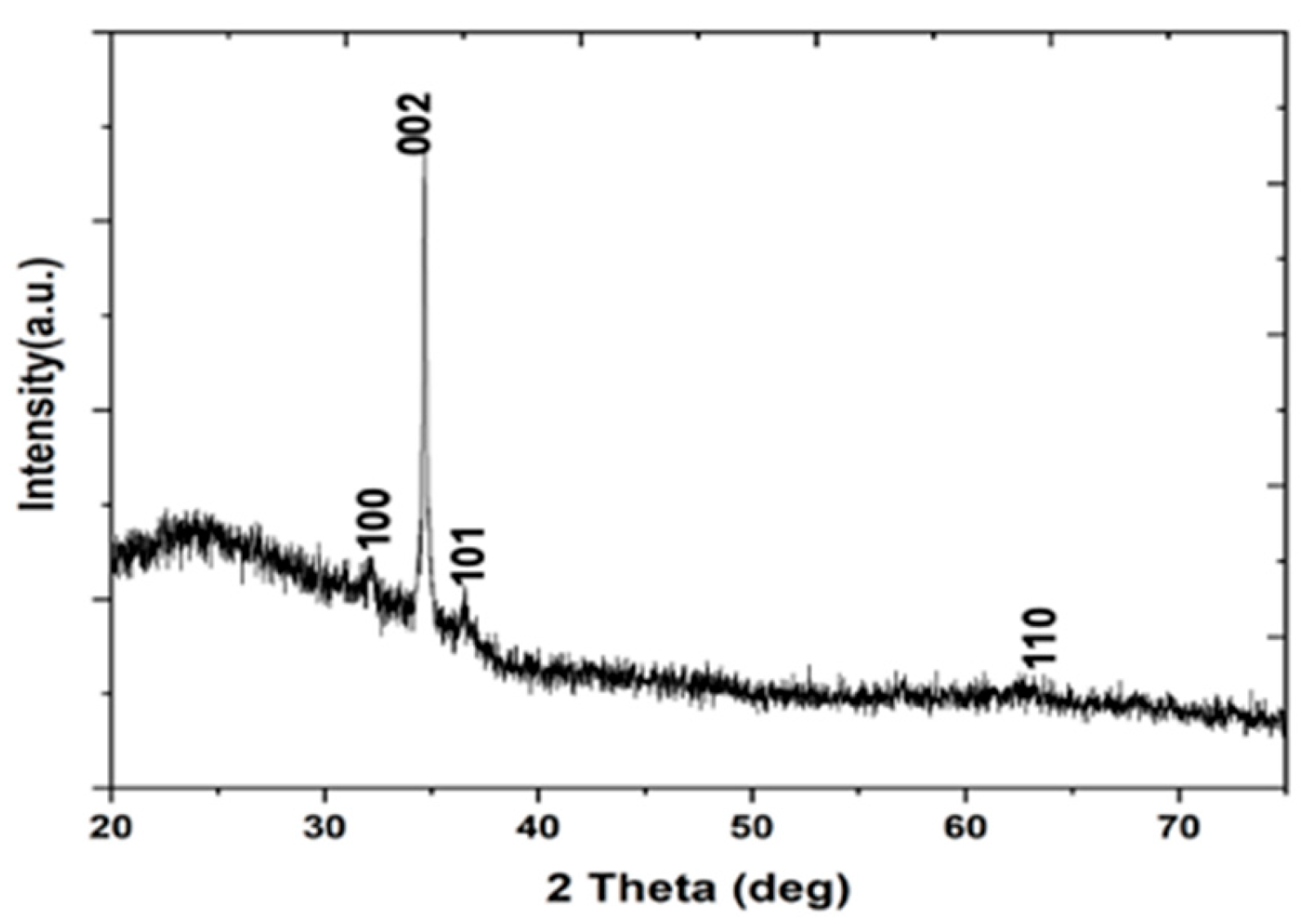
3.2. XRD Results of ZnO Nanoparticles
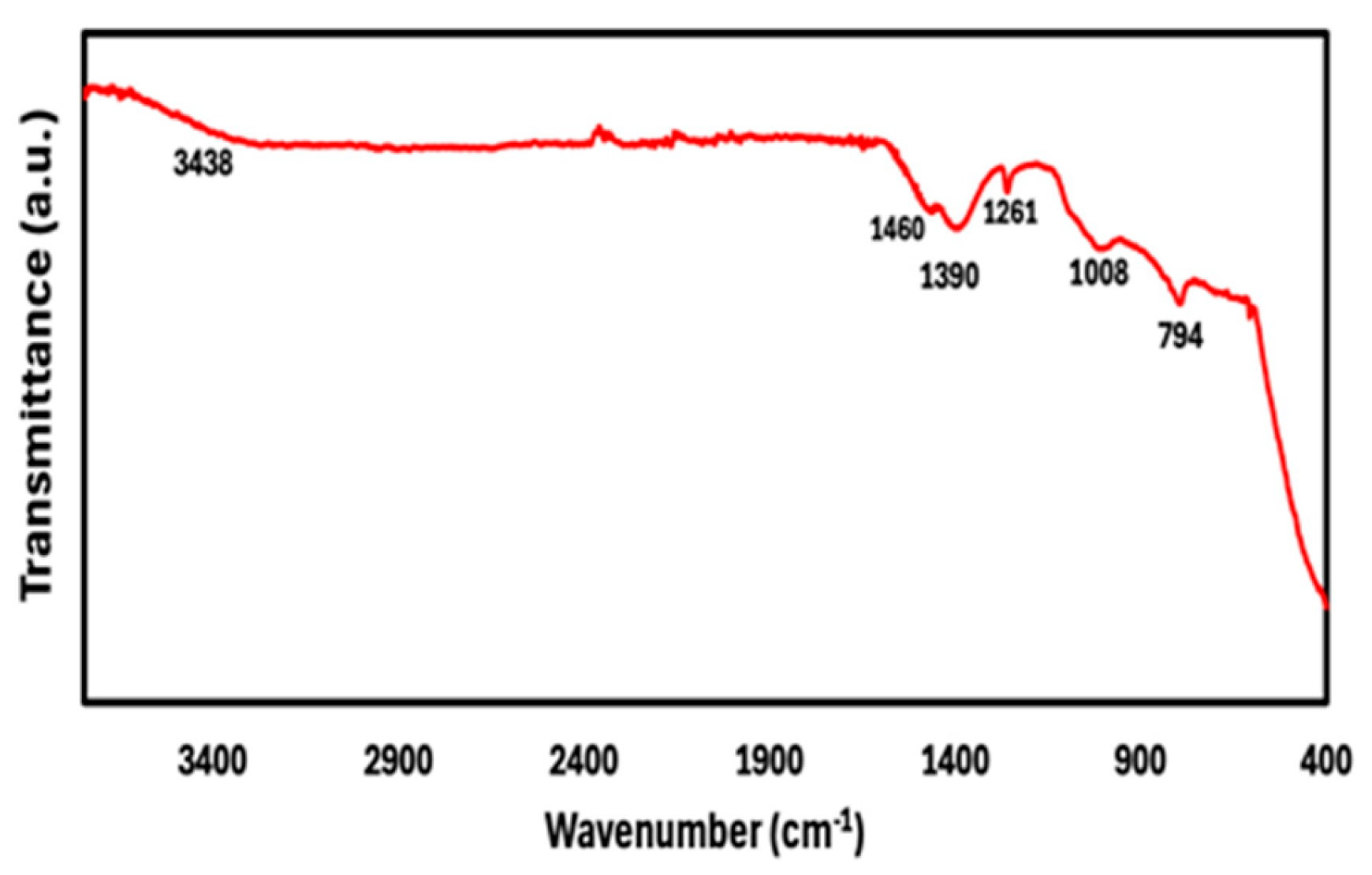
3.3. FTIR Results of Mo-Doped ZnO Nanoparticles
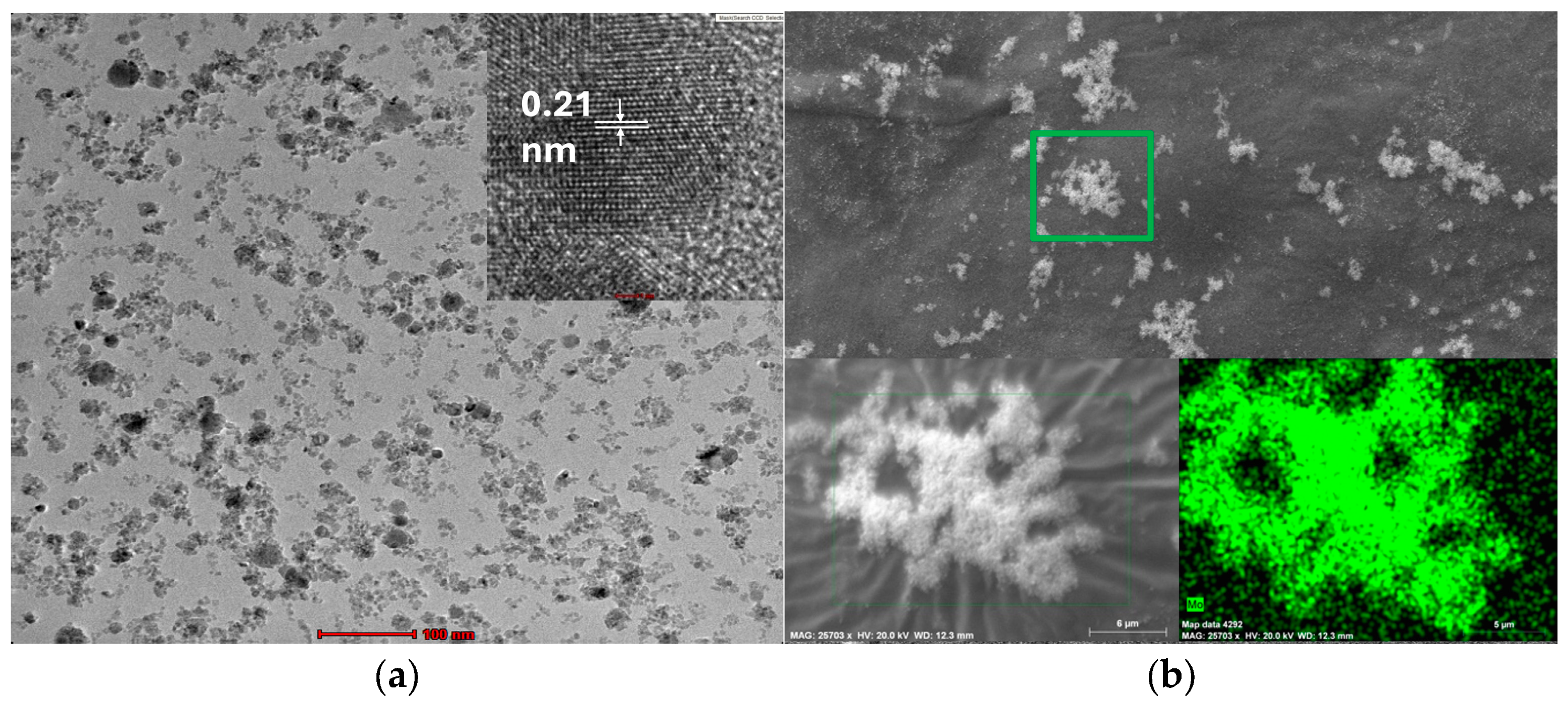
3.4. TEM, Hr-TEM, and SEM Images of ZnO and Mo Nanoparticles Obtained with the Laser Ablation Process
3.5. Photocatalytic Degradation of Mo Nps, ZnO Nps, and Mo-Doped ZnO Nps
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sohaib, M.; Iqbal, T.; Afsheen, S.; Tahir, M.B.; Masood, A.; Rafique, M.; Riaz, K.N.; Sayed, M.A.; El-Rehim, A.F.A.; Ali, A.M. Novel solgel synthesis of Mo-doped ZnO-NPs for photo-catalytic waste water treatment using the RhB dye as a model pollutant. Environment. Dev. Sustain. 2023, 25, 11583–11598. [Google Scholar] [CrossRef]
- Mintcheva, N.; Aljulaih, A.A.; Wunderlich, W.; Kulinich, S.A.; Iwamori, S. Laser-Ablated ZnO Nanoparticles and Their Photocatalytic Activity toward Organic Pollutants. Materials 2018, 11, 1127. [Google Scholar] [CrossRef] [PubMed]
- Gouvêa, C.A.K.; Wypych, F.; Moraes, S.G.; Durán, N.; Nagata, N.; Peralta-Zamora, P. Semiconductor-assisted photocatalytic degradation of reactive dyes in aqueous solution. Chemosphere 2000, 40, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Hsu, Y.; Chen, Y.; Lee, B.; Hwang, J.; Chen, L.; Chen, K. Cobalt-Phosphate-Assisted Photoelectrochemical Water Oxidation by Arrays of Molybdenum-Doped Zinc Oxide Nanorods. ChemSusChem 2014, 7, 2748–2754. [Google Scholar] [CrossRef] [PubMed]
- Tsuzuki, T.; He, R.; Dodd, A.; Saunders, M. Challenges in determining the location of dopants, to study the influence of metal doping on the photocatalytic activities of ZnO nanopowders. Nanomaterials 2019, 9, 481. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wei, J.; Yang, D.; Song, Y.; Yang, Y. Mo-doped ZnO NPs with NIR light enhance peroxidase-like nanozymes and trigger photothermal for bacteria eradication. J. Mater. Chem. C 2025, 13, 3567–3577. [Google Scholar] [CrossRef]
- Parangusan, H.; Ponnamma, D.; Al-Maadeed, M.A.A.; Marimuthu, A. Nanoflower-like yttrium-doped ZnO photocatalyst for the degradation of methylene blue dye. Photochem. Photobiol. 2018, 94, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Jiang, Z.; Berk, D. Molybdenum doped graphene/TiO2 hybrid photocatalyst for UV/visible photocatalytic applications. Sol. Energy 2018, 162, 420–430. [Google Scholar] [CrossRef]
- Samadi, M.; Zirak, M.; Naseri, A.; Khorashadizade, E.; Moshfegh, A.Z. Recent progress on doped ZnO nanostructures for visible-light photocatalysis. Thin Solid Film. 2016, 605, 2–19. [Google Scholar] [CrossRef]
- Gündoğdu, Y.; Kepceoğlu, A.; Gezgin, S.Y.; Küçükçelebi, H.; Kılıç, H.Ş. Femtosecond laser ablation synthesis of nanoparticles and nano-hybrides in ethanol medium. Mater. Today Proc. 2019, 18, 1803–1810. [Google Scholar] [CrossRef]
- Kohzadi, S.; Maleki, A.; Bundschuh, M.; Vahabzadeh, Z.; Johari, S.A.; Rezaee, R.; Shahmoradi, B.; Marzban, N.; Amini, N. Doping zinc oxide (ZnO) nanoparticles with molybdenum boosts photocatalytic degradation of Rhodamine b (RhB): Particle characterization, degradation kinetics and aquatic toxicity testing. J. Mol. Liq. 2023, 385, 122412. [Google Scholar] [CrossRef]
- Gündoğdu, Y.; Dursun, S.; Gezgin, S.Y.; Kiliç, H.Ş. Femtosecond laser-induced production of ZnO@ Ag nanocomposites for an improvement in photocatalytic efficiency in the degradation of organic pollutants. Opt. Laser Technol. 2024, 170, 110291. [Google Scholar] [CrossRef]
- Padwal, Y.; Chauhan, R.; Panchang, R.; Fouad, H.; Gosavi, S.W. Exploring Mo-ZnO@ NF for hydrogen generation and methylene blue remediation: Sunlight-driven catalysis. Front. Phys. 2024, 12, 1416563. [Google Scholar] [CrossRef]
- Sunil Kumar, K.C.; Chandra, S.; Lakshmi Ranganatha, V.; Shivaganga, G.S.; Soundarya, T.L.; Nagaraju, G.; Mallikarjunaswamy, C. An effective approach to improve photocatalytic dye degradation and electrochemical properties of MoO3 nanoparticles. Ionics 2024, 30, 3679–3690. [Google Scholar] [CrossRef]
- Rungsawang, T.; Krobthong, S.; Paengpan, K.; Kaewtrakulchai, N.; Manatura, K.; Eiad-Ua, A.; Boonruang, C.; Wongrerkdee, S. Synergy of functionalized activated carbon and ZnO nanoparticles for enhancing photocatalytic degradation of methylene blue and carbaryl. Radiat. Phys. Chem. 2024, 223, 111924. [Google Scholar] [CrossRef]



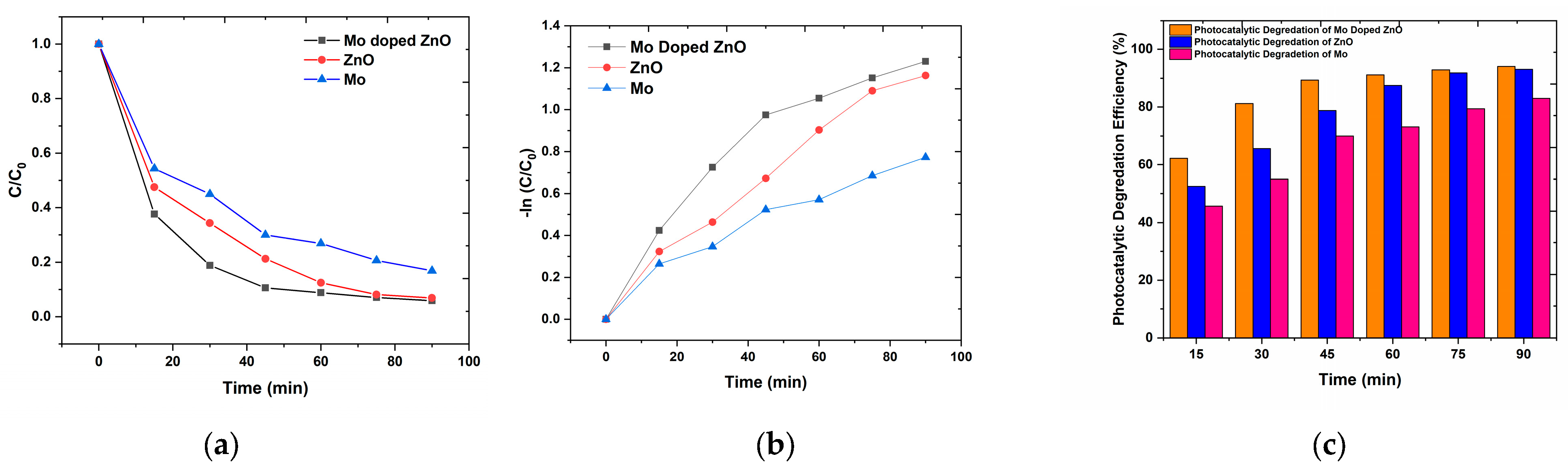
| Photocatalyst | Kinetic Constants, k (min−1) | Photocatalytic Degradation Efficiency (% in 90 min) |
|---|---|---|
| Mo Nps | 0.008 | 83.125 |
| ZnO Nps | 0.013 | 93.125 |
| Mo doped ZnO Nps | 0.014 | 94.110 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kabakcı, Y.G.; Gezgin, S.Y.; Kılıç, H.Ş. Investigation of Laser-Based Mo-Doped ZnO Nanoparticle Production and Photocatalysis Application. Eng. Proc. 2025, 104, 50. https://doi.org/10.3390/engproc2025104050
Kabakcı YG, Gezgin SY, Kılıç HŞ. Investigation of Laser-Based Mo-Doped ZnO Nanoparticle Production and Photocatalysis Application. Engineering Proceedings. 2025; 104(1):50. https://doi.org/10.3390/engproc2025104050
Chicago/Turabian StyleKabakcı, Yasemin Gündoğdu, Serap Yiğit Gezgin, and Hamdi Şükür Kılıç. 2025. "Investigation of Laser-Based Mo-Doped ZnO Nanoparticle Production and Photocatalysis Application" Engineering Proceedings 104, no. 1: 50. https://doi.org/10.3390/engproc2025104050
APA StyleKabakcı, Y. G., Gezgin, S. Y., & Kılıç, H. Ş. (2025). Investigation of Laser-Based Mo-Doped ZnO Nanoparticle Production and Photocatalysis Application. Engineering Proceedings, 104(1), 50. https://doi.org/10.3390/engproc2025104050






