Synthesis of 2-aminopyridine Lactones and Studies of Their Antioxidant, Antibacterial and Antifungal Properties †
Abstract
:1. Introduction
2. Antioxidant Effects
3. Antifungal and Antibacterial Activities
4. Experimental
- (A)
- Synthesis and Screening of Antioxidant Potential
- (B)
- Screening of antibacterial and antifungal properties of the compounds.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salhi, F.; Cheikh, N.; Villemin, D.; Mostefa-Kara, B.; Bar, N.; Jarsalé, K.; Choukchou-Braham, N.; Jarsal, K. Catalyzed reaction of enaminonitrile with primary amines by SbF3: Synthesis of new 2-aminosubstituted-pyridine-fused δ-lactones. Arkivoc 2018, 2018, 65–74. [Google Scholar] [CrossRef]
- Tepe, B.; Daferera, D.; Sokmen, A.; Sokmen, M.; Polissiou, M. Antimicrobial and antioxidant activities of the essential oil and various extracts of Salvia tomentosa Miller (Lamiaceae). Food Chem. 2005, 90, 333–340. [Google Scholar] [CrossRef]
- Shen, Q.; Zhang, B.; Xu, R.; Wang, Y.; Ding, X.; Li, P. Antioxidant activity in vitro of the selenium-contained protein from the Se-enriched Bifidobacterium animalis 01. Anaerobe 2010, 16, 380–386. [Google Scholar] [CrossRef] [PubMed]
- Remmal, A.; Bouchikhi, T.; Rhayour, K.; Ettayebi, M.; Tantaoui-Elaraki, A. Improved Method for the Determination of Antimicrobial Activity of Essential Oils in Agar Medium. J. Essent. Oil Res. 1993, 5, 179–184. [Google Scholar] [CrossRef]
- Biondi, D.; Cianci, P.; Geraci, C.; Ruberto, G.; Piattelli, M. Antimicrobial activity and chemical composition of essential oils from sicilian aromatic plants. Flavour Fragr. J. 1993, 8, 331–337. [Google Scholar] [CrossRef]



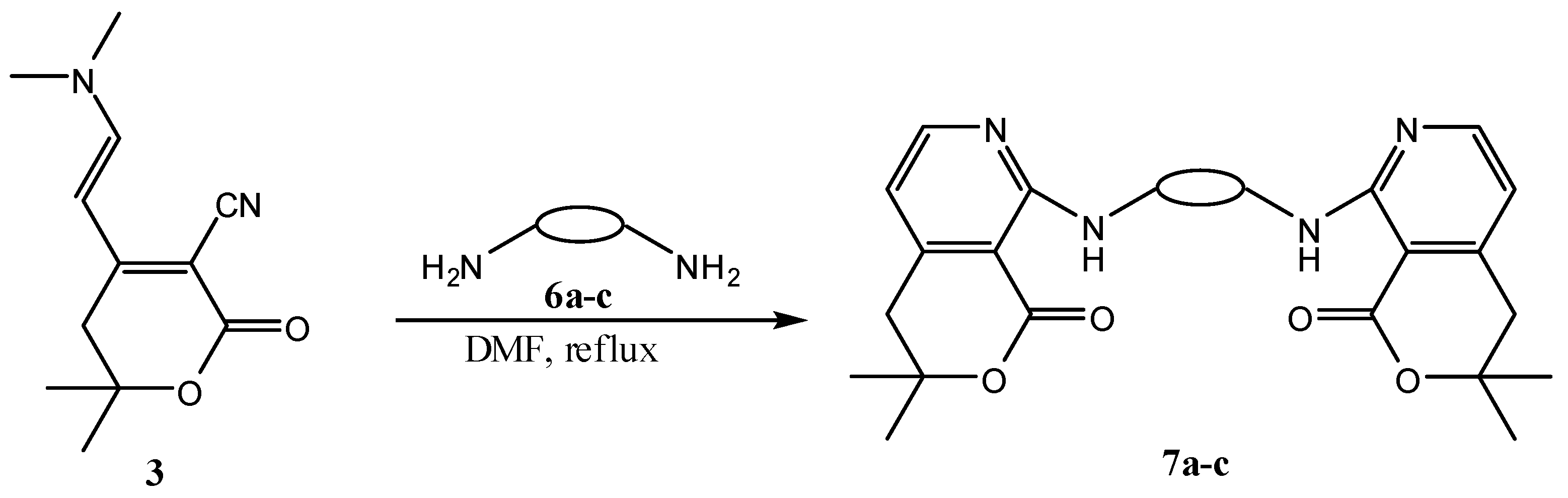
| Entry | Enaminolactone | RNH2 | Product | Yield (%) |
|---|---|---|---|---|
| 1 | 3 | 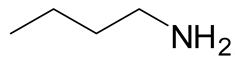 4a 4a |  5a 5a | 95 |
| 2 | 3 |  4b 4b | 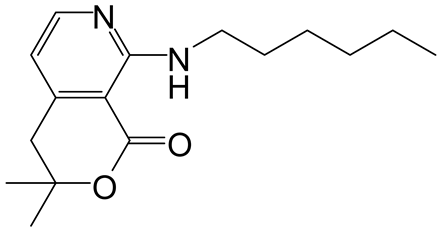 5b 5b | 87 |
| 3 | 3 | 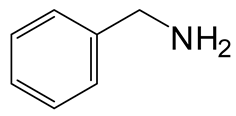 4c 4c |  5c 5c | 92 |
| 4 | 3 | 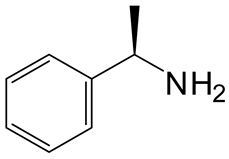 4d 4d | 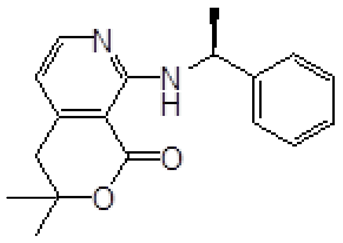 5d 5d | 96 |
| 5 | 3 | 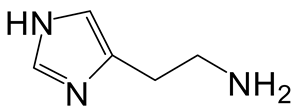 4e 4e | 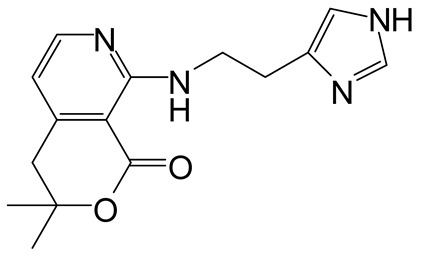 5e 5e | 95 |
| 6 | 3 | 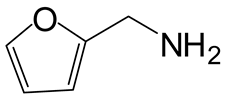 4f 4f | 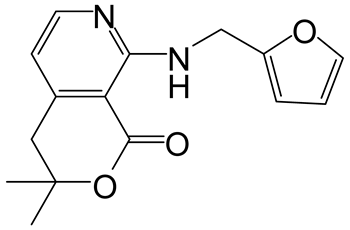 5f 5f | 96 |
| Entry | RNH2 | Product | Yield (%) | |
|---|---|---|---|---|
| 1 | 3 | 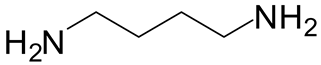 6a 6a | 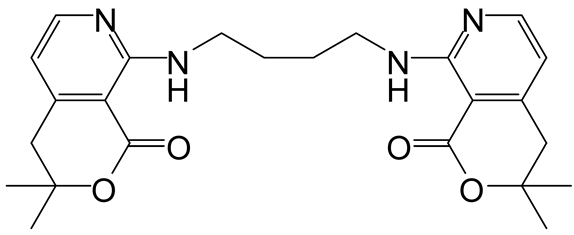 7a 7a | 57 |
| 2 | 3 | 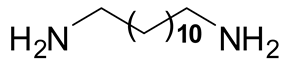 6b 6b | 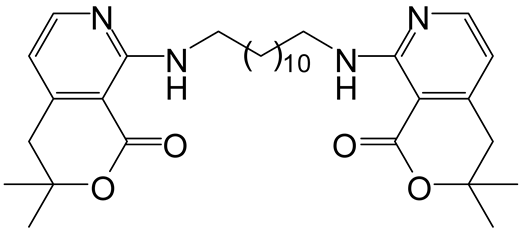 7b 7b | 60 |
| 3 | 3 | 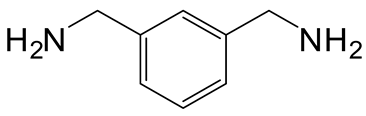 6c 6c |  7c 7c | 89 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salhi, F.; Cheikh, N.; Villemin, D.; Bar, N. Synthesis of 2-aminopyridine Lactones and Studies of Their Antioxidant, Antibacterial and Antifungal Properties. Chem. Proc. 2022, 8, 94. https://doi.org/10.3390/ecsoc-25-11709
Salhi F, Cheikh N, Villemin D, Bar N. Synthesis of 2-aminopyridine Lactones and Studies of Their Antioxidant, Antibacterial and Antifungal Properties. Chemistry Proceedings. 2022; 8(1):94. https://doi.org/10.3390/ecsoc-25-11709
Chicago/Turabian StyleSalhi, Fadila, Nawel Cheikh, Didier Villemin, and Nathalie Bar. 2022. "Synthesis of 2-aminopyridine Lactones and Studies of Their Antioxidant, Antibacterial and Antifungal Properties" Chemistry Proceedings 8, no. 1: 94. https://doi.org/10.3390/ecsoc-25-11709
APA StyleSalhi, F., Cheikh, N., Villemin, D., & Bar, N. (2022). Synthesis of 2-aminopyridine Lactones and Studies of Their Antioxidant, Antibacterial and Antifungal Properties. Chemistry Proceedings, 8(1), 94. https://doi.org/10.3390/ecsoc-25-11709






