Synthesis of Heterocycles and Nucleosides Forming Higher—Order Structures †
Abstract
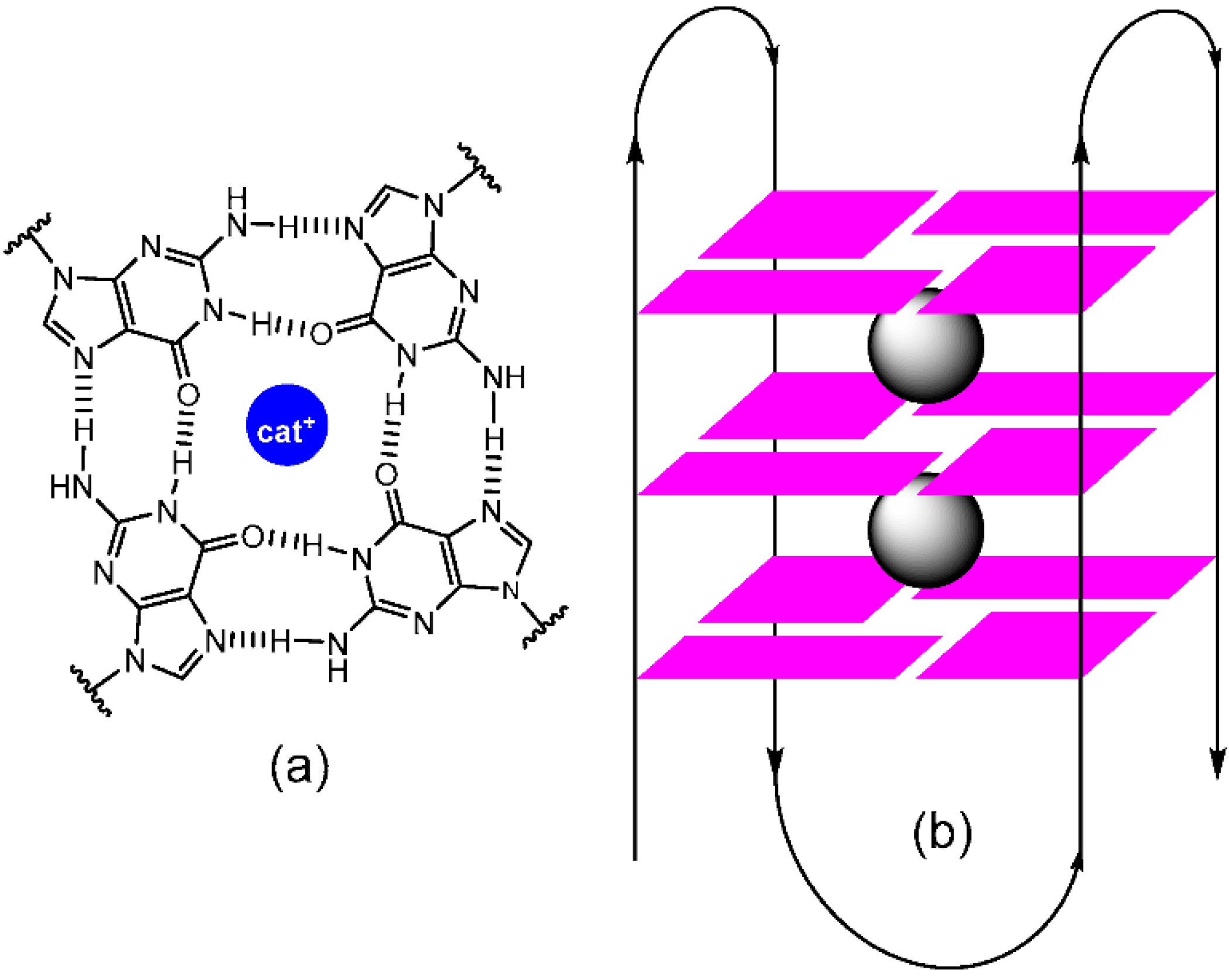
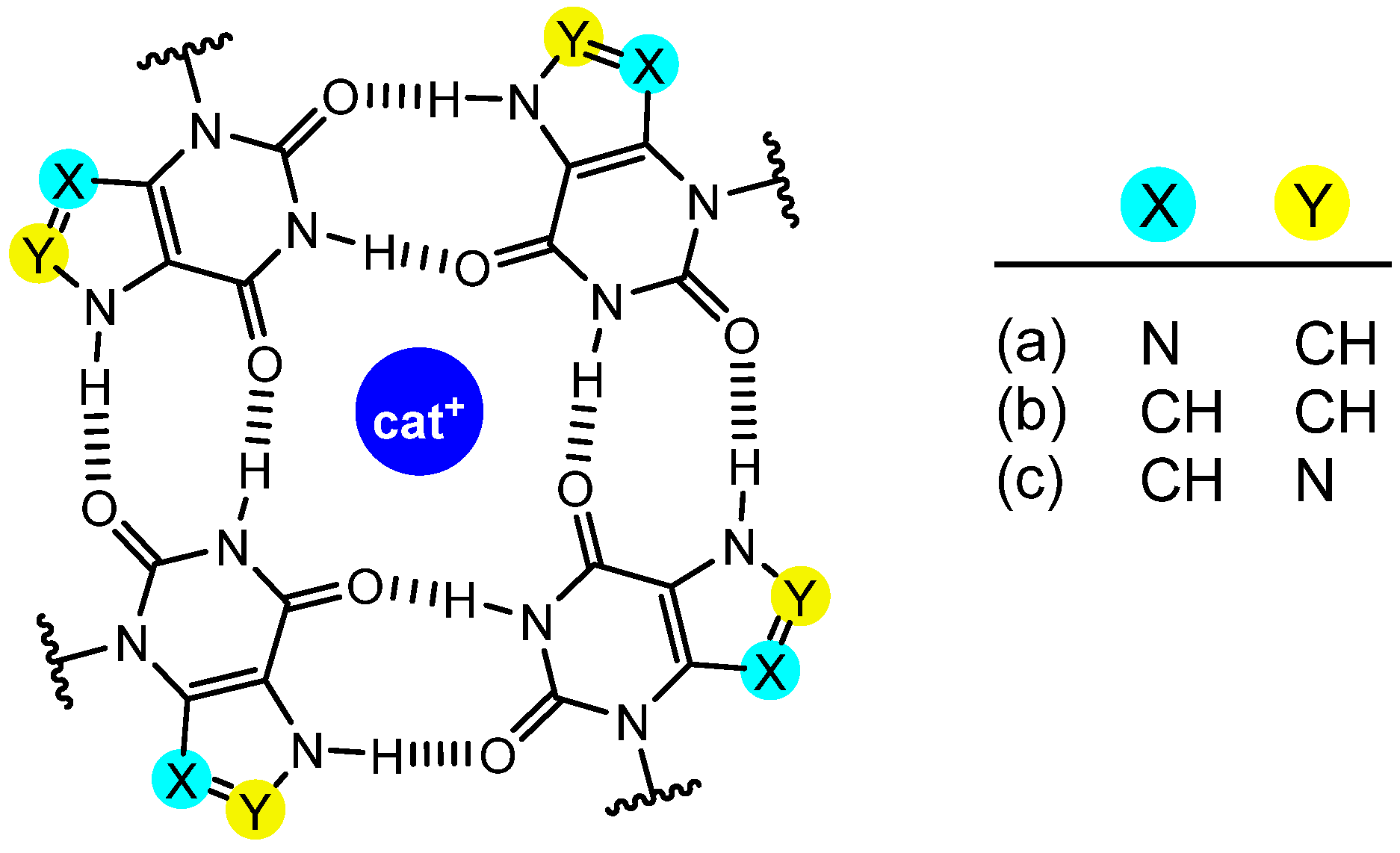
1. Introduction
2. Chemical Synthesis
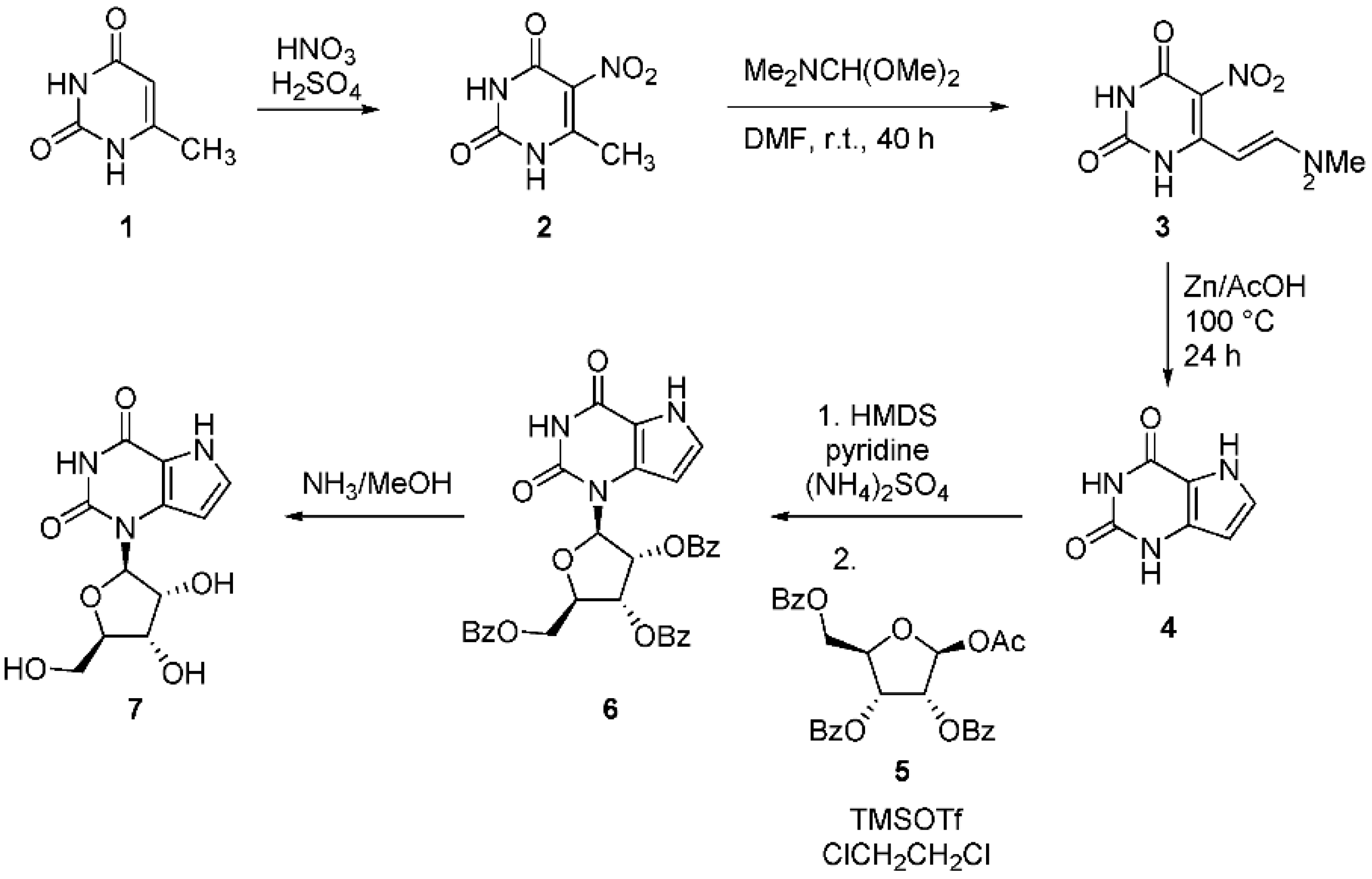
2.1. 9-Deazaxanthines
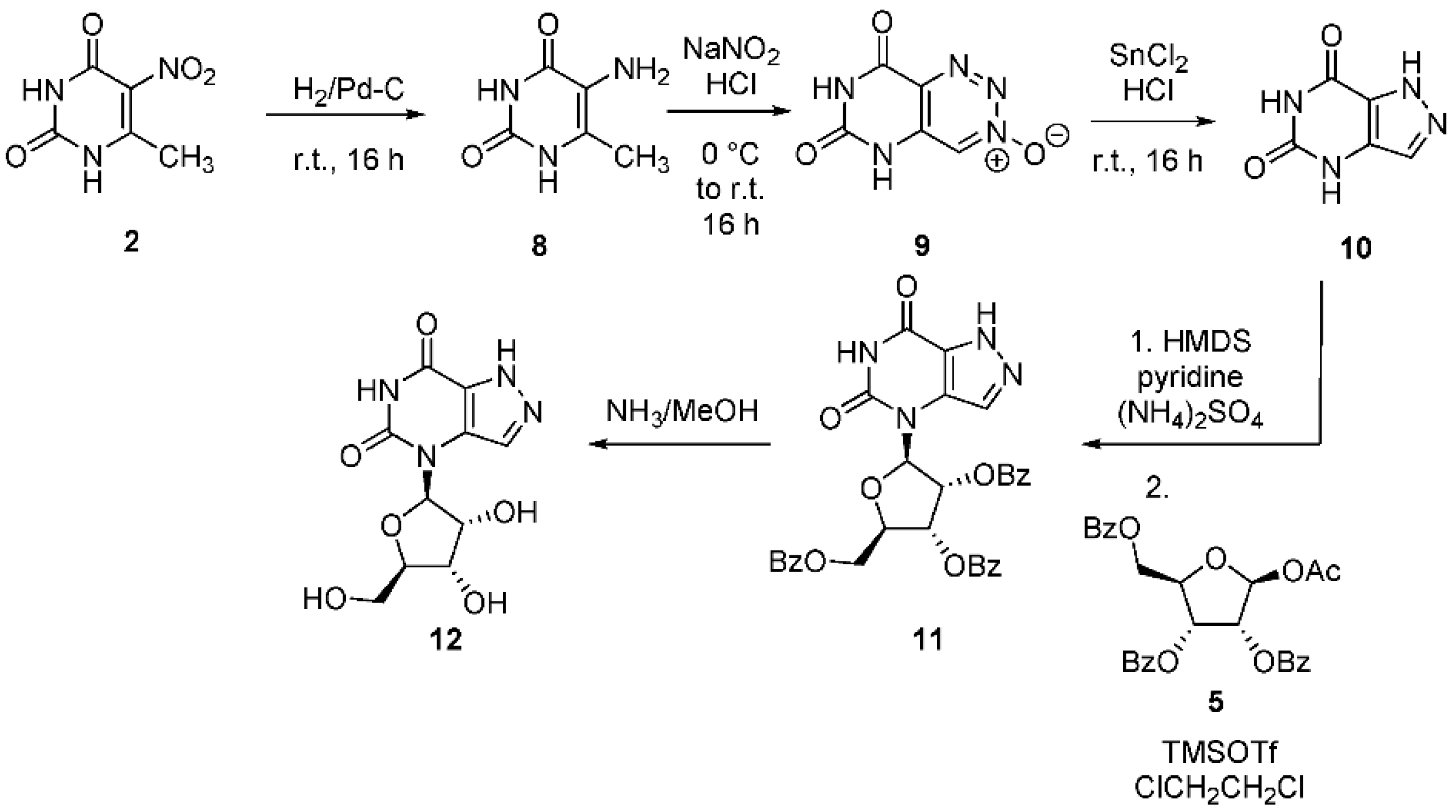
2.2. 8-Aza-9-Deazaxanthines
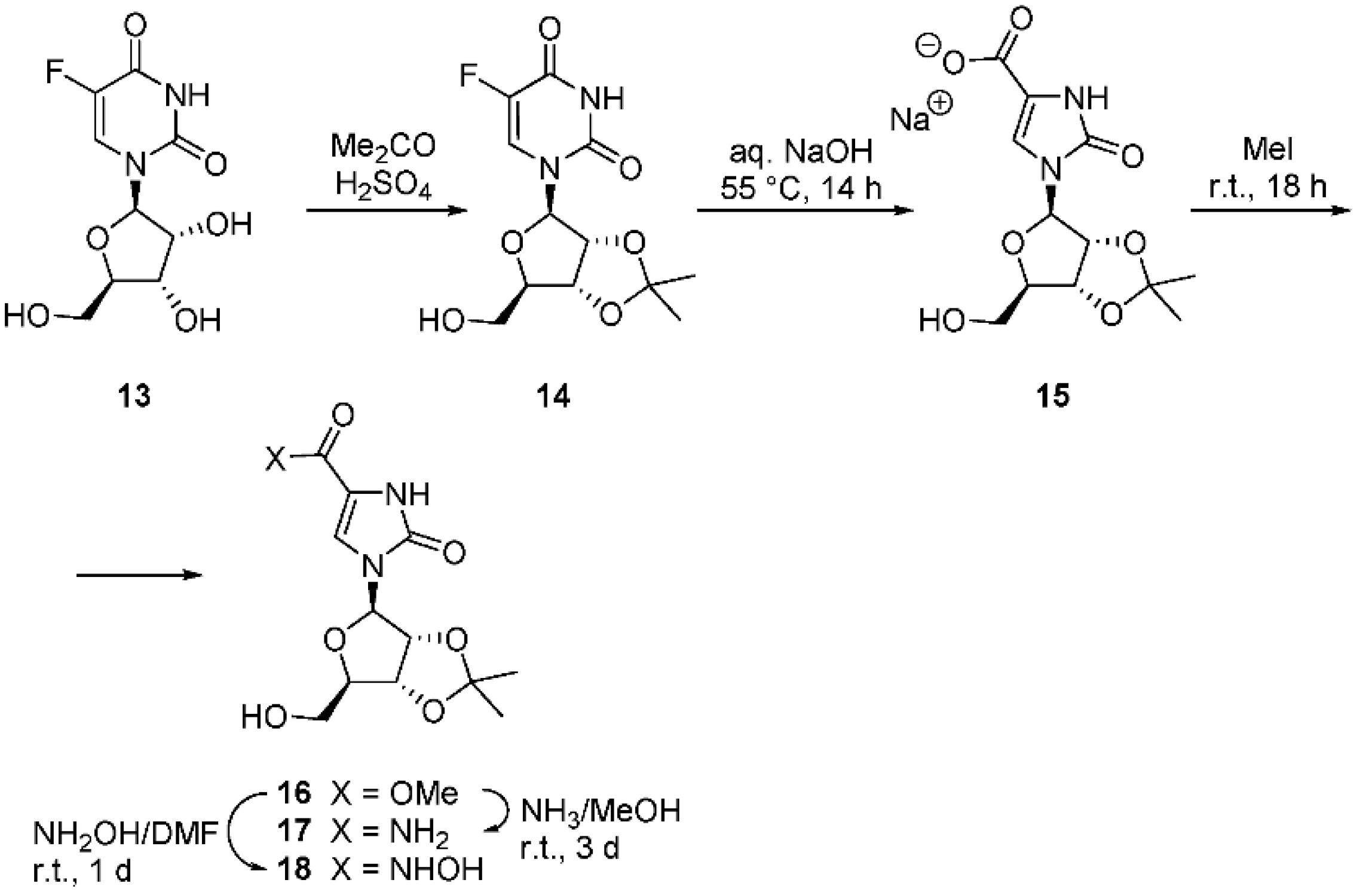
2.3. Imidazolidinone Carboxylic Acid Nucleoside Derivatives
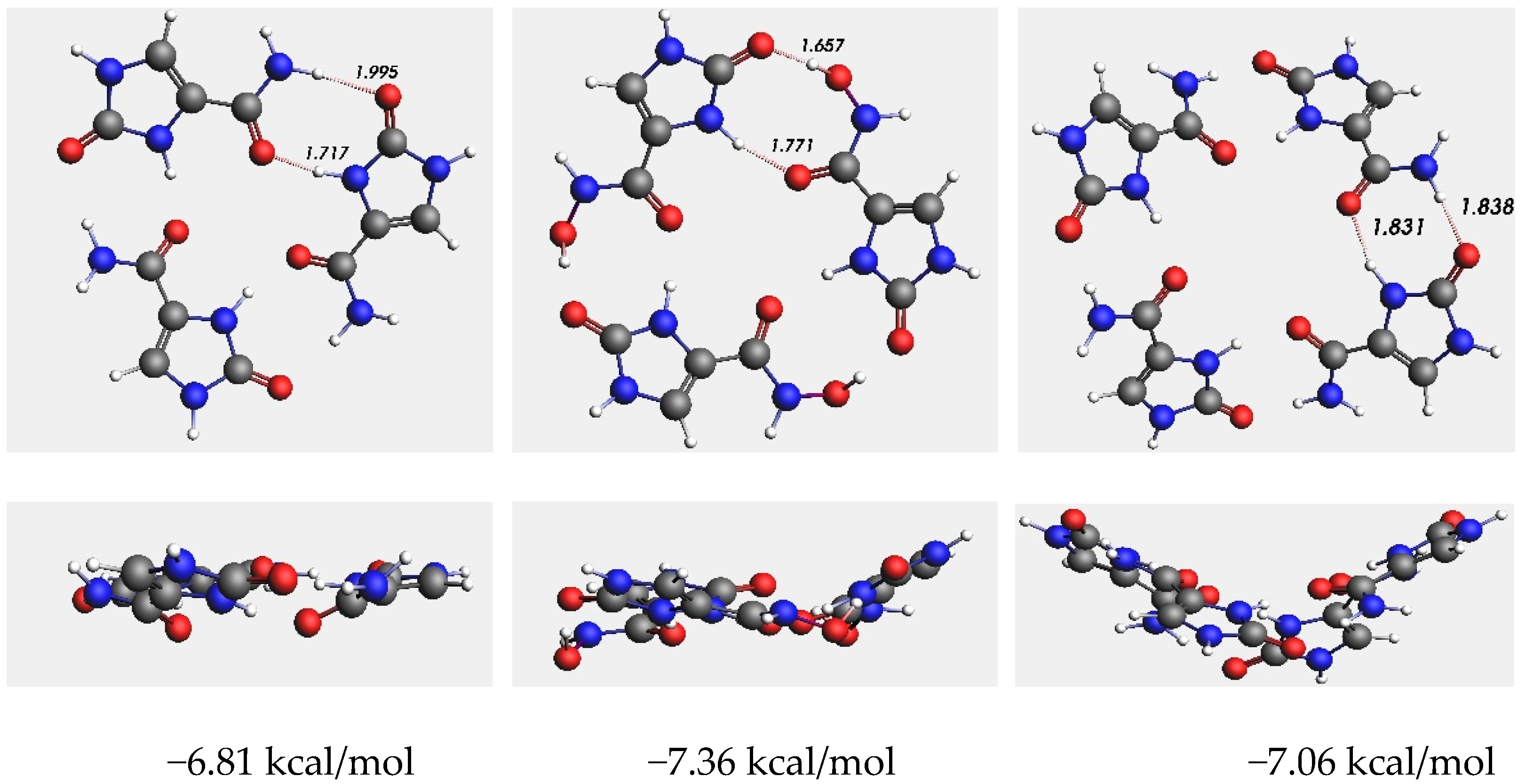
3. Computational Studies of Imidazolidinones
4. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Neidle, S.; Balasubramanian, S. Quadruplex Nucleic Acids; The Royal Society of Chemistry: Cambridge, UK, 2006. [Google Scholar]
- McKenzie, L.K.; El-Khoury, R.; Thorpe, J.D.; Damha, M.J.; Hollenstein, M. Recent progress in non-native nucleic acid modifications. Chem. Soc. Rev. 2021, 50, 5126–5164. [Google Scholar] [CrossRef] [PubMed]
- Mergny, J.-L.; De Cian, A.; Ghelab, A.; Saccà, B.; Lacroix, L. Kinetics of tetramolecular quadruplexes. Nucleic Acids Res. 2005, 33, 81–94. [Google Scholar] [CrossRef] [PubMed]
- Largy, E.; Mergny, J.-L.; Gabelica, V. The Alkali Metal Ions: Their Role for Life; Sigel, A., Sigel, H., Sigel, O.R.K., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 203–258. [Google Scholar] [CrossRef]
- Zaccaria, F.; Paragi, G.; Fonseca Guerra, C. The role of alkali metal cations in the stabilization of guanine quadruplexes: Why K+ is the best. Phys. Chem. Chem. Phys. 2016, 18, 20895–20904. [Google Scholar] [CrossRef] [PubMed]
- Paragi, G.; Kupihár, Z.; Endre, G.; Fonseca Guerra, C.; Kovács, L. The evaluation of 5-amino- and 5-hydroxyuracil derivatives as potential quadruplex-forming agents. Org. Biomol. Chem. 2017, 15, 2174–2184. [Google Scholar] [CrossRef] [PubMed]
- Szolomájer, J.; Paragi, G.; Batta, G.; Fonseca Guerra, C.; Bickelhaupt, F.M.; Kele, Z.; Pádár, P.; Kupihár, Z.; Kovács, L. 3-Substituted xanthines as promising candidates for quadruplex formation: Computational, synthetic and analytical studies. New J. Chem. 2011, 35, 476–482. [Google Scholar] [CrossRef]
- Ciesielski, A.; Haar, S.; Bényei, A.; Paragi, G.; Fonseca Guerra, C.; Bickelhaupt, F.M.; Masiero, S.; Szolomájer, J.; Samorì, P.; Spada, G.P.; et al. Self-Assembly of N3-Substituted Xanthines in the Solid State and at the Solid–Liquid Interface. Langmuir 2013, 29, 7283–7290. [Google Scholar] [CrossRef] [PubMed]
- Ciesielski, A.; Haar, S.; Paragi, G.; Kupihár, Z.; Kele, Z.; Masiero, S.; Fonseca Guerra, C.; Bickelhaupt, F.M.; Spada, G.P.; Kovács, L.; et al. Supramolecular H-bonded porous networks at surfaces: Exploiting primary and secondary interactions in a bi-component melamine–xanthine system. Phys. Chem. Chem. Phys. 2013, 15, 12442–12446. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cawrse, B.M.; Lapidus, R.S.; Cooper, B.; Choi, E.Y.; Seley-Radtke, K.L. Anticancer properties of halogenated pyrrolo[3,2-d]pyrimidines with decreased toxicity via N5 substitution. ChemMedChem 2018, 13, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Rao, T.S.; Revankar, G.R.; Vinayak, R.S.; Robins, R.K. Synthesis of 5,7-disubstituted-4-β-D-ribofuranosylpyrazolo[4,3-d]-pyrimidines and 2,4-disubstituted-1-β-D-ribofuranosylpyrrolo[3,2-d]-pyrimidines as congeners of uridine and cytidine. J. Heterocycl. Chem. 1992, 29, 343–354. [Google Scholar] [CrossRef]
- Papesch, V.; Dodson, R.M. Pyrimido[5,4-d][l,2,3]triazines. J. Org. Chem. 1963, 28, 1329–1331. [Google Scholar] [CrossRef]
- Papesch, V.; Dodson, R.M. Isomeric pyrazoIo[4,3-d]pyrimidinediones. J. Org. Chem. 1965, 30, 199–203. [Google Scholar] [CrossRef]
- Fox, J.J. Pyrimidine nucleoside transformations via anhydronucleosides. Pure Appl. Chem. 1969, 18, 223–256. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Váradi, Z.; Paragi, G.; Kupihár, Z.; Kele, Z.; Kovács, L. Synthesis of Heterocycles and Nucleosides Forming Higher—Order Structures. Chem. Proc. 2022, 8, 4. https://doi.org/10.3390/ecsoc-25-11705
Váradi Z, Paragi G, Kupihár Z, Kele Z, Kovács L. Synthesis of Heterocycles and Nucleosides Forming Higher—Order Structures. Chemistry Proceedings. 2022; 8(1):4. https://doi.org/10.3390/ecsoc-25-11705
Chicago/Turabian StyleVáradi, Zoltán, Gábor Paragi, Zoltán Kupihár, Zoltán Kele, and Lajos Kovács. 2022. "Synthesis of Heterocycles and Nucleosides Forming Higher—Order Structures" Chemistry Proceedings 8, no. 1: 4. https://doi.org/10.3390/ecsoc-25-11705
APA StyleVáradi, Z., Paragi, G., Kupihár, Z., Kele, Z., & Kovács, L. (2022). Synthesis of Heterocycles and Nucleosides Forming Higher—Order Structures. Chemistry Proceedings, 8(1), 4. https://doi.org/10.3390/ecsoc-25-11705





