Multicomponent Reactions of Isocyanides for the Preparation of Low Molecular Weight Gelators: Preliminary Studies †
Abstract
:1. Introduction
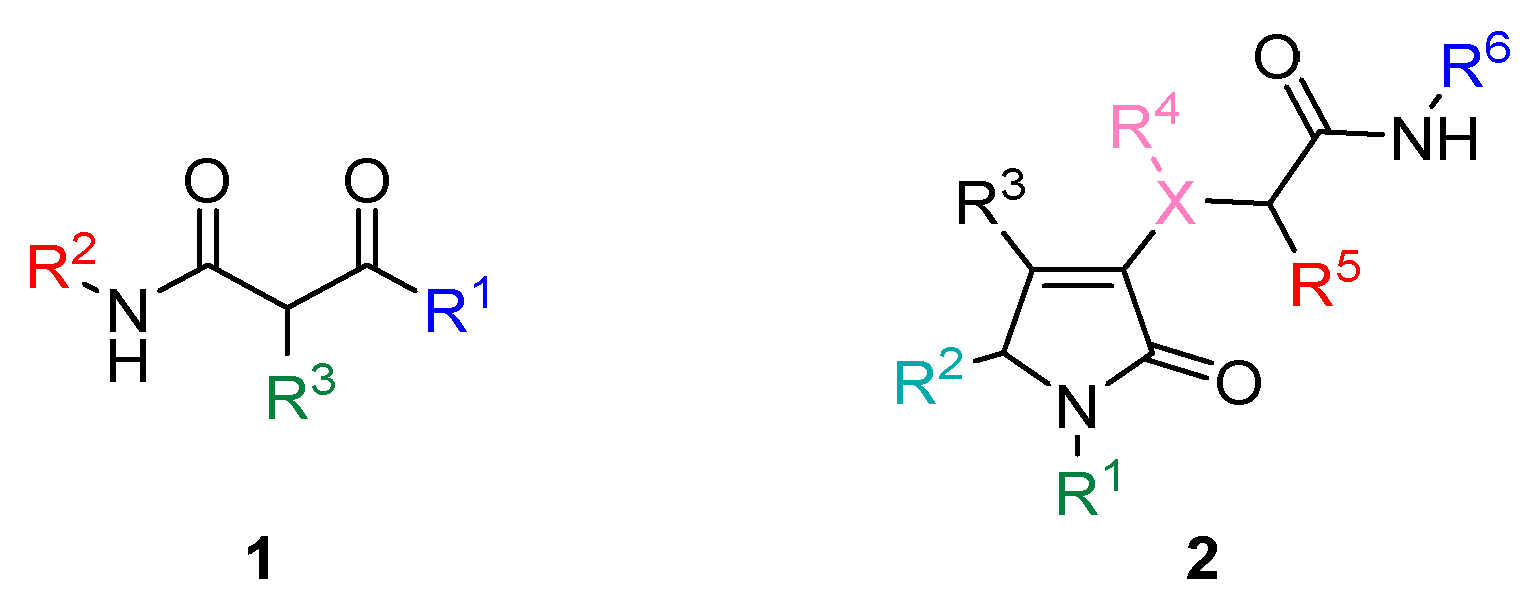
2. Results and Discussion
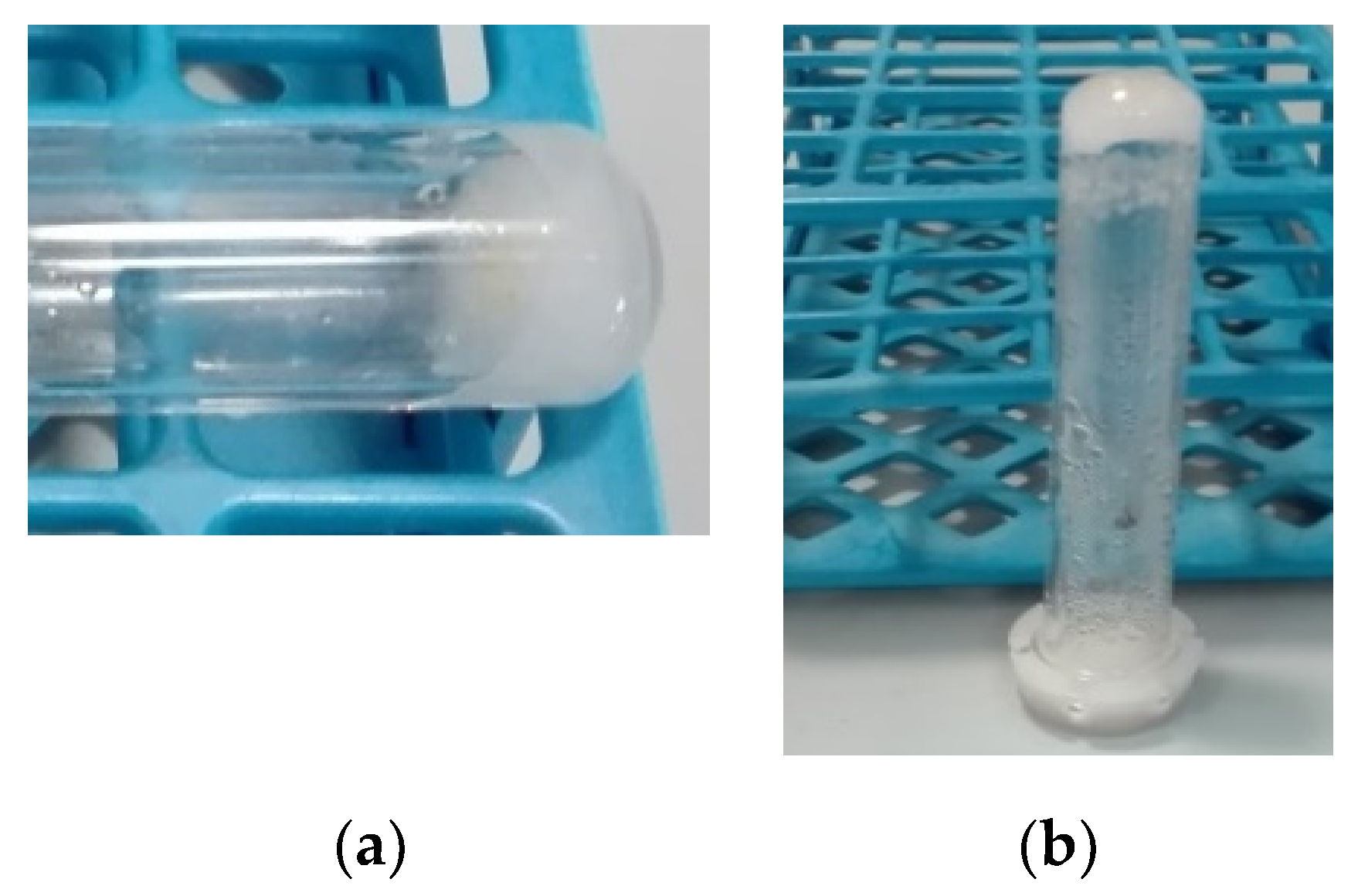
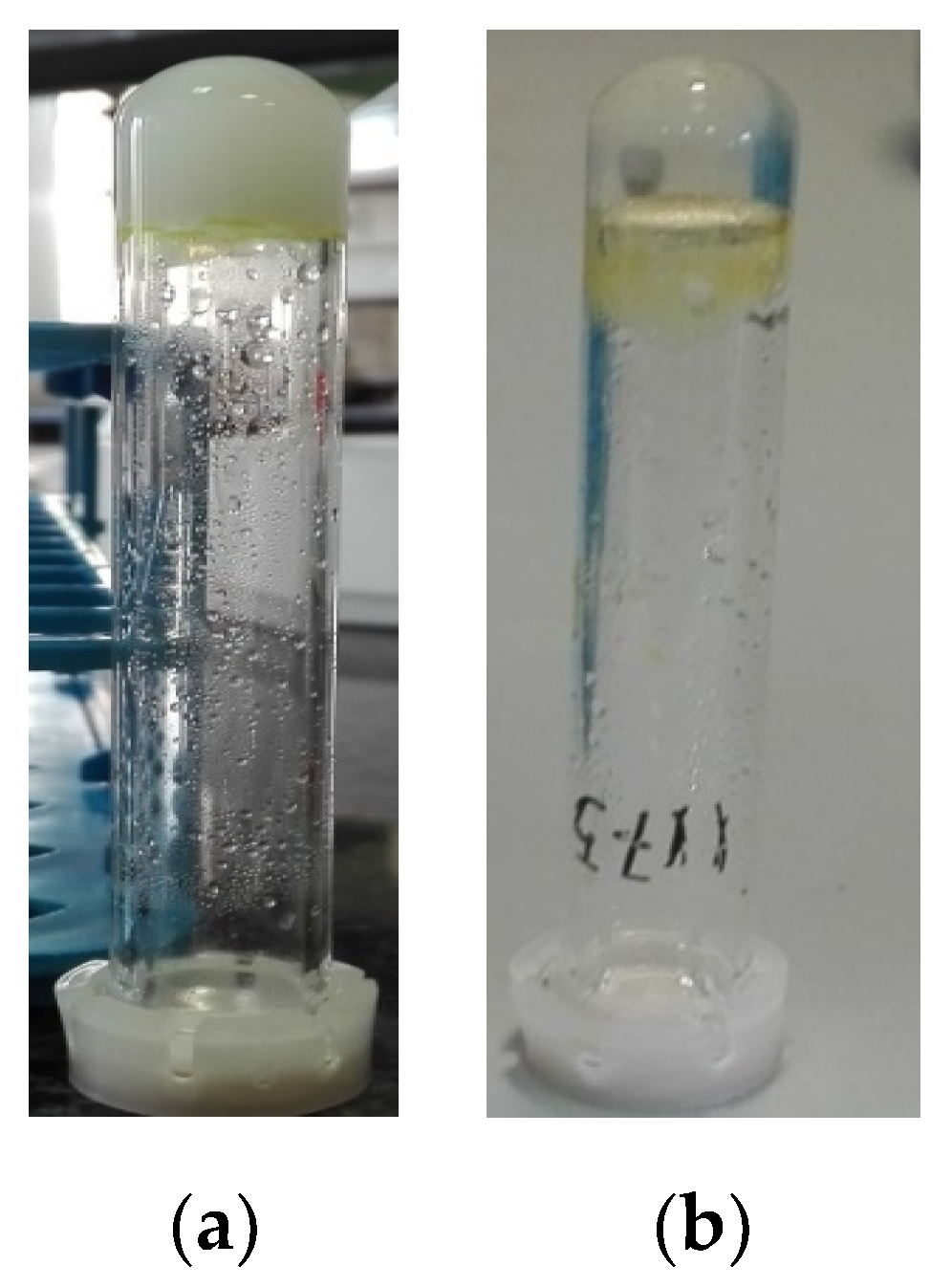
2.1. Self-Assembling Properties of Dicarbonyl Compounds
2.2. Self-Assembling Properties of Enol-Ugi and Enol-Passerini Adducts
3. Experimental
3.1. Materials and Methods
3.2. Synthesis of Compounds Tested as Gelators
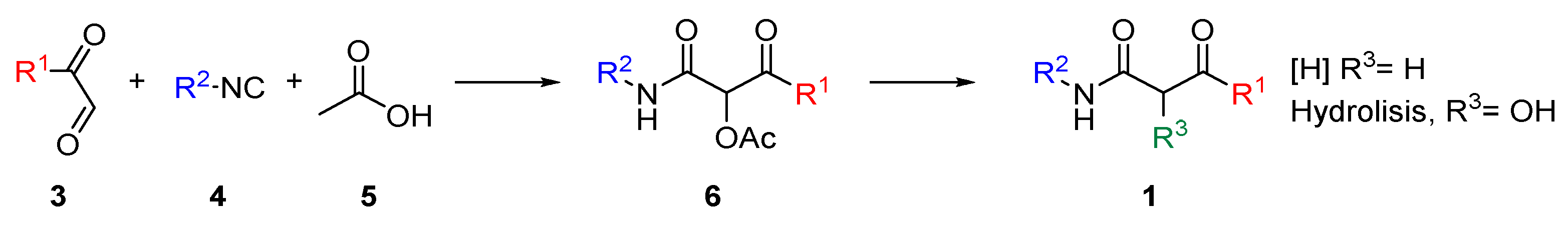
3.2.1. Synthesis of Dicarbonylic Compounds
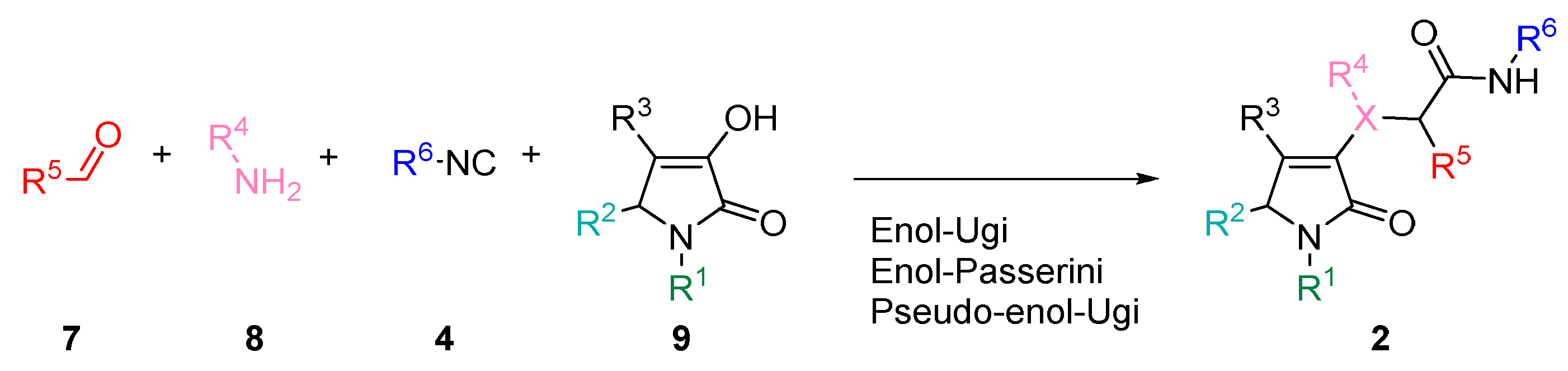
3.2.2. Synthesis of Enol-Ugi and Enol-Passerini Adducts
3.3. Gelation Protocol
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Terech, P.; Weiss, R.G. Low Molecular Mass Gelators of Organic Liquids and the Properties of Their Gels. Chem. Rev. 1997, 97, 3133–3160. [Google Scholar] [CrossRef] [PubMed]
- de Loos, M.; Feringa, B.L.; van Esch, J.H. Design and Application of Self-Assembled Low Molecular Weight Hydrogels. Eur. J. Org. Chem. 2005, 2005, 3615–3631. [Google Scholar] [CrossRef]
- George, M.; Weiss, R.G. Molecular Organogels. Soft Matter Comprised of Low-Molecular-Mass Organic Gelators and Organic Liquids†. Acc. Chem. Res. 2006, 39, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ouyang, G.; Niu, D.; Sang, Y. Supramolecular gelatons: Towards the design of molecular gels. Org. Chem. Front. 2018, 5, 2885–2900. [Google Scholar] [CrossRef]
- Hirst, A.R.; Escuder, B.; Miravet, J.F.; Smith, D.K. High-Tech Applications of Self-Assembling Supramolecular Nanostructured Gel-Phase Materials: From Regenerative Medicine to Electronic Devices. Angew. Chem. Int. Ed. Engl. 2008, 47, 8002–8018. [Google Scholar] [CrossRef]
- Du, X.; Zhou, J.; Shi, J.; Xu, B. Supramolecular Hydrogelators and Hydrogels: From Soft Matter to Molecular Biomaterials. Chem. Rev. 2015, 115, 13165–13307. [Google Scholar] [CrossRef]
- Kim, J.; Park, Y.; Tae, G.; Lee, K.B.; Hwang, C.M.; Hwang, S.J.; Kim, I.S.; Noh, I.; Sun, K. Characterization of low-molecular-weight hyaluronic acid-based hydrogel and differential stem cell responses in the hydrogel microenvironments. J. Biomed. Mater. Res. A 2009, 88A, 967–975. [Google Scholar] [CrossRef]
- Yang, Z.; Liang, G.; Guo, Z.; Guo, Z.; Xu, B. Intracellular Hydrogelation of Small Molecules Inhibits Bacterial Growth. Angew. Chem. Int. Ed. Engl. 2007, 46, 8216–8219. [Google Scholar] [CrossRef]
- Ganesh, S.D.; Saha, N.; Zandraa, O.; Zuckermann, R.N.; Sáha, P. Peptoids and polypeptoids: Biomimetic and bioinspired materials for biomedical applications. Polym. Bull. 2017, 74, 3455–3466. [Google Scholar] [CrossRef]
- Cai, Y.; Zheng, C.; Xiong, F.; Ran, W.; Zhai, Y.; Zhu, H.H.; Wang, H.; Li, Y.; Zhang, P. Recent Progress in the Design and Application of Supramolecular Peptide Hydrogels in Cancer Therapy. Adv. Healthc. Mater. 2021, 10, 2001239. [Google Scholar] [CrossRef]
- Bursavich, M.G.; Rich, D.H. Designing Non-Peptide Peptidomimetics in the 21st Century: Inhibitors Targeting Conformational Ensembles. J. Med. Chem. 2002, 45, 541–558. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.A.; Steed, J.W. Exploiting Cavities in Supramolecular Gels. Angew. Chem. Int. Ed. Engl. 2010, 49, 6718–6724. [Google Scholar] [CrossRef] [PubMed]
- Lau, K.H.A. Peptoids for biomaterials science. Biomater. Sci. 2014, 2, 627–633. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomasini, C.; Castellucci, N. Peptides and peptidomimetics that behave as low molecular weight gelators. Chem. Soc. Rev. 2013, 42, 156–172. [Google Scholar] [CrossRef]
- Sauvée, C.; Ström, A.; Haukka, M.; Sundén, H. A Multi-Component Reaction towards the Development of Highly Modular Hydrogelators. Chem. Eur. J. 2018, 24, 8071–8075. [Google Scholar] [CrossRef]
- Ugi, I.; Meyr, R.; Fetzer, U.; Steinbrückner, C. Versammlungsberichte. Angew. Chem. 1959, 71, 386. [Google Scholar] [CrossRef]
- Mangunuru, H.P.R.; Yang, H.; Wang, G. Synthesis of peptoid based small molecular gelators by a multiple component reaction. Chem. Commun. 2013, 49, 4489–4491. [Google Scholar] [CrossRef]
- Neo, A.G.; Delgado, J.; Polo, C.; Marcaccini, S.; Marcos, C.F. A new synthesis of β-keto amides by reduction of Passerini adducts. Tetrahedron Lett. 2005, 46, 23–26. [Google Scholar] [CrossRef]
- Carrillo, R.M.; Neo, A.G.; López-García, L.; Marcaccini, S.; Marcos, C.F. Zinc catalysed ester solvolysis. Application to the synthesis of tartronic acid derivatives. Green Chem. 2006, 8, 787–789. [Google Scholar] [CrossRef]
- Neo, A.G.; Carrillo, R.M.; Delgado, J.; Marcaccini, S.; Marcos, C.F. A multicomponent approach to the synthesis of 1,3-dicarbonylic compounds. Mol. Divers. 2011, 15, 529–539. [Google Scholar] [CrossRef]
- Castellano, T.G.; Neo, A.G.; Marcaccini, S.; Marcos, C.F. Enols as Feasible Acid Components in the Ugi Condensation. Org. Lett. 2012, 14, 6218–6221. [Google Scholar] [CrossRef] [PubMed]
- Neo, A.G.; Marcos, C.F. Selective Synthesis of 3-Substituted Pyrrolidinones by Enol-Passerini and Anomalous Enol-Passerini Condensations. Org. Lett. 2018, 20, 3875–3878. [Google Scholar] [CrossRef] [PubMed]
- Van Lommel, R.; De Borggraeve, W.M.; De Proft, F.; Alonso, M. Computational Tools to Rationalize and Predict the Self-Assembly Behavior of Supramolecular Gels. Gels 2021, 7, 87. [Google Scholar] [CrossRef] [PubMed]


| Entry | Compound | H2O:EtOH | c (mg/mL) | Gelation |
|---|---|---|---|---|
| 1 | 1a | 1:0.1 | 12 | NO |
| 2 | 1b | 1:0.1 | 5 | NO |
| 3 | 1c | 1:1 | 14 | NO |
| 4 | 1c | 1:0.1 | 6 | NO |
| 6 | 1d | 1:0.1 | 6 | NO |
| 5 | 1e | 1:0.1 | 12 | NO |
| 7 | 1f | 1:0.1 | 9 | PARTIAL |
| 8 | 1g | 1:0.1 | 8 | YES |
| 9 | 1h | 1:0.1 | 9 | NO |
| Entry | Compound | H2O:EtOH | c (mg/mL) | Gelation |
|---|---|---|---|---|
| 1 | 2a | 1:0.2 | 10 | NO |
| 2 | 2b | 1:0.2 | 12 | NO |
| 3 | 2c | 1:0.2 | 12 | NO |
| 4 | 2d | 1:0.2 | 14 | NO |
| 5 | 2e | 1:0.2 | 12 | YES |
| 6 | 2f | 1:0.2 | 10 | NO |
| 7 | 2g | 1:0.2 | 10 | NO |
| 9 | 2h | 1:0.2 | 11 | FILM |
| 10 | 2i | 1:0.2 | 8 | NO |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramiro, J.L.; Marcos, C.F.; Neo, A.G. Multicomponent Reactions of Isocyanides for the Preparation of Low Molecular Weight Gelators: Preliminary Studies. Chem. Proc. 2022, 8, 1. https://doi.org/10.3390/ecsoc-25-11668
Ramiro JL, Marcos CF, Neo AG. Multicomponent Reactions of Isocyanides for the Preparation of Low Molecular Weight Gelators: Preliminary Studies. Chemistry Proceedings. 2022; 8(1):1. https://doi.org/10.3390/ecsoc-25-11668
Chicago/Turabian StyleRamiro, José L., Carlos F. Marcos, and Ana G. Neo. 2022. "Multicomponent Reactions of Isocyanides for the Preparation of Low Molecular Weight Gelators: Preliminary Studies" Chemistry Proceedings 8, no. 1: 1. https://doi.org/10.3390/ecsoc-25-11668
APA StyleRamiro, J. L., Marcos, C. F., & Neo, A. G. (2022). Multicomponent Reactions of Isocyanides for the Preparation of Low Molecular Weight Gelators: Preliminary Studies. Chemistry Proceedings, 8(1), 1. https://doi.org/10.3390/ecsoc-25-11668






