Multicomponent Crystalline Solid Forms of Pyridinecarboxamides and DL-2-Hydroxy-2-phenylacetic Acid †
Abstract
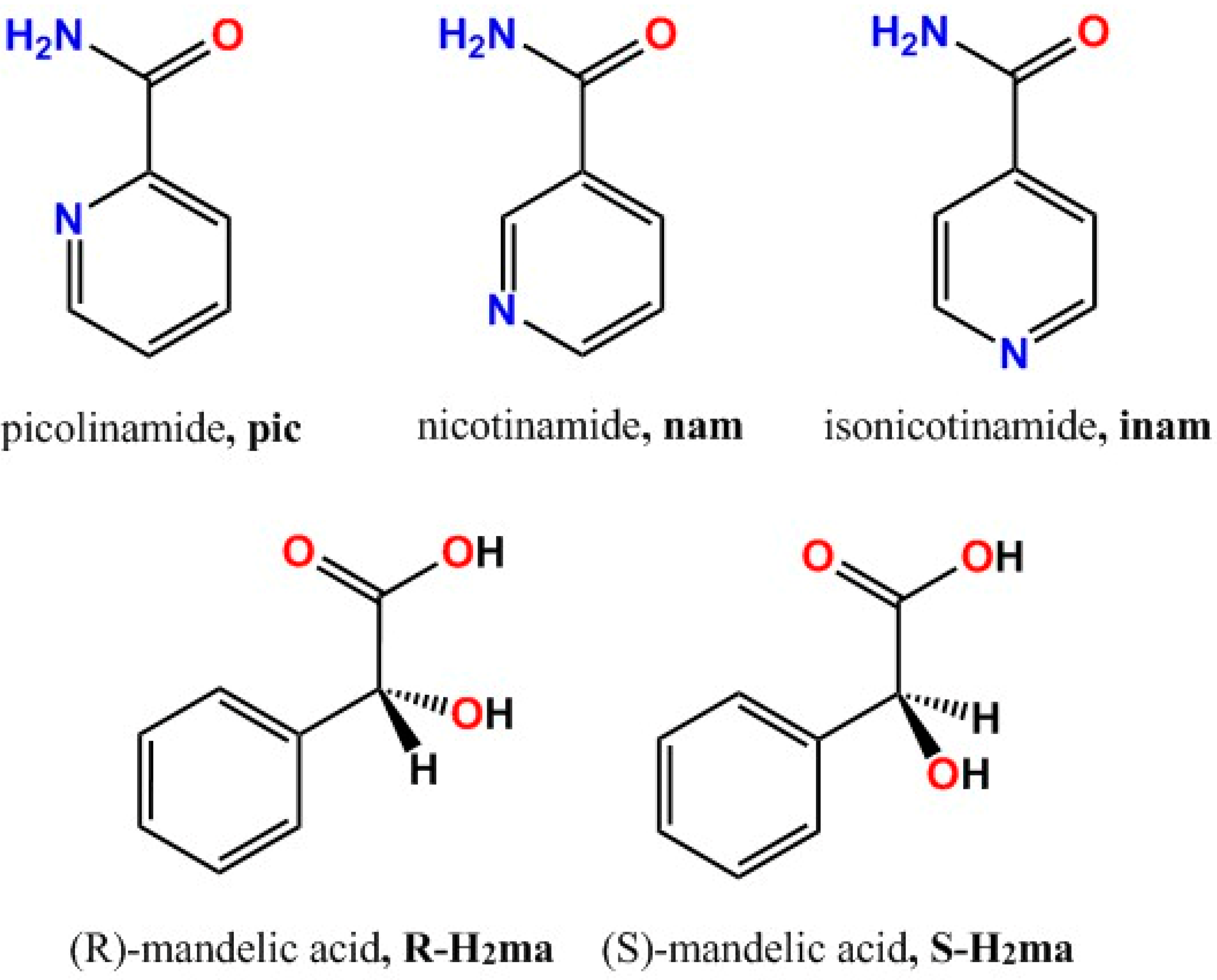
:1. Introduction
2. Materials and Methods
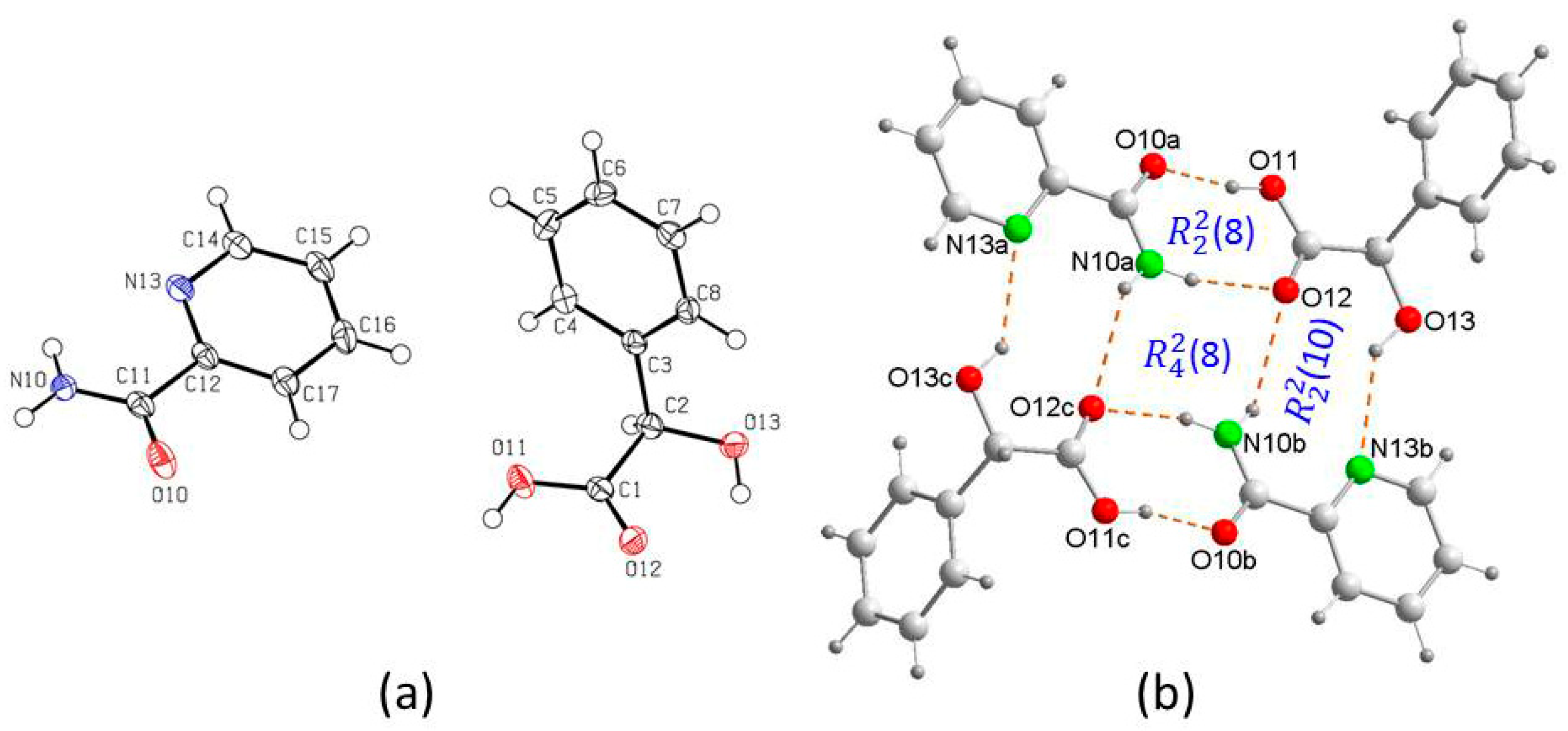
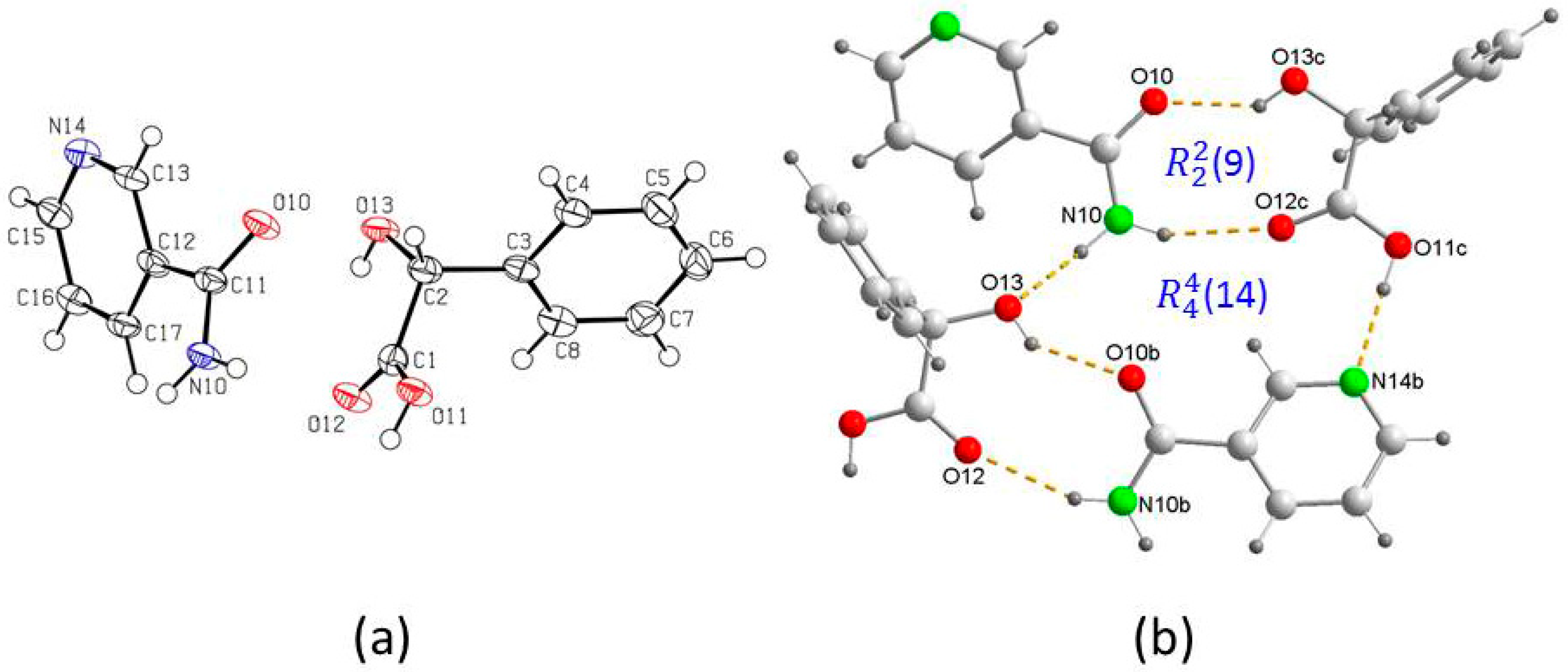
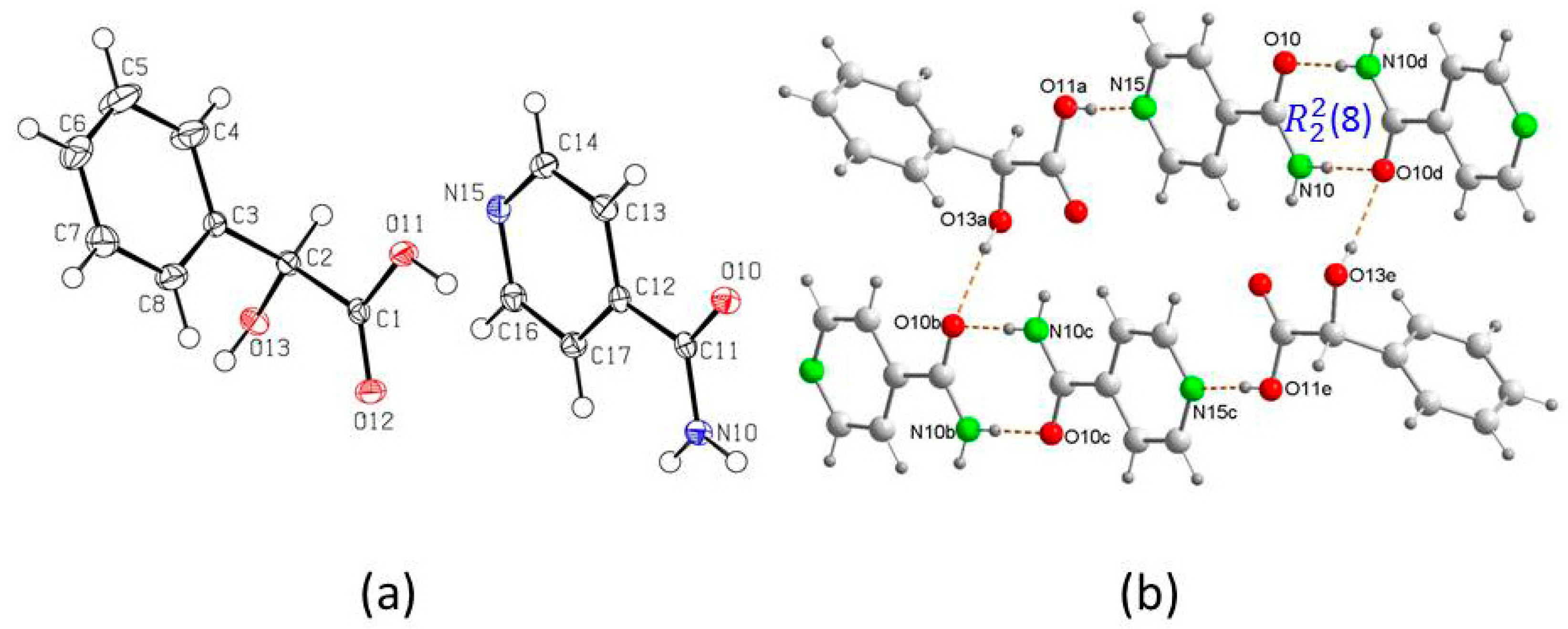
3. Results and Discussion
Crystal Structure Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nangia, A.K.; Desiraju, G.R. Crystal Engineering: An Outlook for the Future. Angew. Chem. Int. Ed. 2019, 58, 4100–4107. [Google Scholar] [CrossRef] [PubMed]
- Desiraju, G.R.; Vittal, J.J.; Ramanan, A. Crystal Engineering: A Text Book; World Scientific: Singapore, 2011. [Google Scholar]
- Desiraju, G.R. Crystal Engineering: From Molecule to Crystal. J. Am. Chem. Soc. 2013, 135, 9952–9967. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Jafari, M.; Padrela, L.; Walker, G.M.; Croker, D.M. Creating Cocrystals: A Review of Pharmaceutical Cocrystal Preparation Routes and Applications. Cryst. Growth Des. 2018, 18, 6370–6387. [Google Scholar] [CrossRef]
- Berry, D.J.; Seaton, C.C.; Clegg, W.; Harrington, R.W.; Coles, S.J.; Horton, P.N.; Hursthouse, M.B.; Storey, R.; Jones, W.; Friščić, T.; et al. Applying Hot-Stage Microscopy to Co-Crystal Screening: A Study of Nicotinamide with Seven Active Pharmaceutical Ingredients. Cryst. Growth Des. 2008, 8, 1697–1712. [Google Scholar] [CrossRef]
- Aakeröy, C.B.; Beatty, A.M.; Helfrich, B.A. A High-Yielding Supramolecular Reaction. J. Am. Chem. Soc. 2002, 124, 14425–14432. [Google Scholar] [CrossRef] [PubMed]
- Sarma, B.; Reddy, L.S.; Nangia, A. The Role of π-Stacking in the Composition of Phloroglucinol and Phenazine Cocrystals. Cryst. Growth Des. 2008, 8, 4546–4552. [Google Scholar] [CrossRef]
- Shattock, T.R.; Arora, K.K.; Vishweshwar, P.; Zaworotko, M.J. Hierarchy of Supramolecular Synthons: Persistent Carboxylic Acid···Pyridine Hydrogen Bonds in Cocrystals That also Contain a Hydroxyl Moiety. Cryst. Growth Des. 2008, 8, 4533–4545. [Google Scholar] [CrossRef]
- Borba, A.; Gómez-Zavaglia, A.; Fausto, R. Molecular Structure, Vibrational Spectra, Quantum Chemical Calculations and Photochemistry of Picolinamide and Isonicotinamide Isolated in Cryogenic Inert Matrixes and in the Neat Low-Temperature Solid Phases. J. Phys. Chem. A 2008, 112, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.A.; Liu, L.; Ghaderi, N.; Johns, A.; Hatcher, M.E.; Mueller, L.J. The Amide Rotational Barriers in Picolinamide and Nicotinamide: NMR and ab Initio Studies. J. Am. Chem. Soc. 2003, 125, 10125–10132. [Google Scholar] [CrossRef] [PubMed]
- Alvarez-Lorenzo, C.; Castiñeiras, A.; Frontera, A.; García-Santos, I.; González-Pérez, J.M.; Niclós-Gutiérrez, J.; Rodríguez-González, I.; Vílchez-Rodríguez, E.; Zaręba, J.K. Recurrent motifs in pharmaceutical cocrystals involving glycolic acid: X-ray characterization, Hirshfeld surface analysis and DFT calculations. CrystEngComm 2020, 22, 6674–6689. [Google Scholar] [CrossRef]
- Van Putten, P.L. Mandelic acid and urinary tract infections. Antonie Leeuwenhoek 1979, 45, 622–623. [Google Scholar] [CrossRef]
- Gamidi, R.K.; Rasmuson, Å.C. Estimation of Melting Temperature of Molecular Cocrystals Using Artificial Neural Network Model. Cryst. Growth Des. 2017, 17, 175–182. [Google Scholar] [CrossRef]
- Kerr, H.E.; Softley, L.K.; Suresh, K.; Hodgkinsona, P.; Evans, I.R. Structure and physicochemical characterization of a naproxen–picolinamide cocrystal. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2017, 73, 168–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhogala, B.R.; Basavoju, S.; Nangia, A. Ape and layer structures in cocrystals of some di- and tricarboxylic acids with 4,4′-bipyridines and isonicotinamide. From binary to ternary cocrystals. CrystEngComm 2005, 7, 551–562. [Google Scholar] [CrossRef]
- Cruz-Cabeza, A.J. Acid–base crystalline complexes and the pKa rule. CrystEngComm 2012, 14, 6362–6365. [Google Scholar] [CrossRef]
- Chan, H.C.S.; Woollam, G.R.; Wagner, T.; Schmidtc, M.U.; Lewis, R.A. Can picolinamide be a promising cocrystal former? CrystEngComm 2014, 16, 4365–4368. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.-W.; Harasimowicz, M.T.; De Villiers, M.M.; Yu, L. Cocrystals of Nicotinamide and (R)-Mandelic Acid in Many Ratios with Anomalous Formation Properties. J. Am. Chem. Soc. 2013, 135, 18981–18989. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castiñeiras, A.; García-Santos, I.; Torres-Iglesias, R. Multicomponent Crystalline Solid Forms of Pyridinecarboxamides and DL-2-Hydroxy-2-phenylacetic Acid. Chem. Proc. 2022, 8, 22. https://doi.org/10.3390/ecsoc-25-11729
Castiñeiras A, García-Santos I, Torres-Iglesias R. Multicomponent Crystalline Solid Forms of Pyridinecarboxamides and DL-2-Hydroxy-2-phenylacetic Acid. Chemistry Proceedings. 2022; 8(1):22. https://doi.org/10.3390/ecsoc-25-11729
Chicago/Turabian StyleCastiñeiras, Alfonso, Isabel García-Santos, and Rocío Torres-Iglesias. 2022. "Multicomponent Crystalline Solid Forms of Pyridinecarboxamides and DL-2-Hydroxy-2-phenylacetic Acid" Chemistry Proceedings 8, no. 1: 22. https://doi.org/10.3390/ecsoc-25-11729
APA StyleCastiñeiras, A., García-Santos, I., & Torres-Iglesias, R. (2022). Multicomponent Crystalline Solid Forms of Pyridinecarboxamides and DL-2-Hydroxy-2-phenylacetic Acid. Chemistry Proceedings, 8(1), 22. https://doi.org/10.3390/ecsoc-25-11729





