Abstract
BODIPY dyes have received great attention in the last few years as optical chemosensors since they can recognize metal ions in solutions through optical signals (colorimetric and/or fluorimetric). In this context, our research group reports the synthesis of a carbazolyl-BODIPY derivative and its respective characterization by 1H NMR spectroscopy and mass spectrometry. Furthermore, a preliminary study of the chemosensory capacity of this BODIPY derivative was carried out in acetonitrile solution in the presence of several anions and a highly selective fluorimetric response was obtained for F-.
1. Introduction
The development of probes capable of recognizing ions with biological and environmental importance through optical signals (colorimetric and/or fluorimetric) is an attractive goal nowadays. Fluoride is an example of an anion harmful to human health at certain levels, since its high intake can lead to chronic diseases such as dental and skeleton fluorosis, and metabolic dysfunctions [1,2]. Thus, simple and rapid sensing tools to monitor fluoride levels in biological samples are of great importance. Boron-dipyrromethene (BODIPY) derivatives have received great attention for chemosensory applications due to their remarkable physical–chemical properties, such as high molar absorption coefficients, high quantum fluorescence yields, intense and narrow absorption/emission bands in the visible region of the electromagnetic spectrum, and good photochemical stability. Additionally, the BODIPY core can be easily functionalized to be used as a selective chromo-fluorogenic chemosensor for various analytes [3,4,5].
In continuation of the work developed in our research group [6,7,8,9,10,11,12], regarding the synthesis and evaluation of the optoelectronic properties of BODIPY derivatives for several applications, namely, as chromofluorogenic chemosensors, we report the synthesis, spectroscopic characterization, and evaluation of the chemosensory ability of a novel BODIPY derivative functionalized with a carbazolyl group at the meso position as a fluorimetric chemosensor for the selective detection of fluoride.
2. Experimental Section
2.1. Methods and Materials
All reagents were purchased from Sigma-Aldrich, Acros, and Fluka and used as received. Thin-layer chromatography (TLC) analysis were carried out on 0.25 mm thick precoated silica plates (Merck Fertigplatten Kieselgel 60F254) and the spots were visualized under UV light. Dry flash chromatography was performed using silica gel 60 with a diameter between 230 and 400 mesh (Merck, Kenilworth, NJ, USA). The 1H NMR spectra were recorded on a Bruker Avance III device at 400 MHz, using the solvent peak as an internal reference. Mass spectrometry analyses were performed at the “C.A.C.T.I.-Unidad de Espectrometria de Masas” at the University of Vigo, Spain. The deuterated solvent used in nuclear magnetic resonance spectroscopy was CDCl3 with a 99.8% deuteration degree, containing 0.03% v/v TMS (Sigma Aldrich, Av. Alm. Gago Coutinho 132, 1950-037 Lisboa, Portugal). The absorption spectra were obtained using a Shimadzu UV/2501PC spectrophotometer and fluorescence spectra were collected using a Horiba FluoroMax-4 spectrofluorometer.
2.2. Synthesis of BODPY Derivative 1
2,4-Dimethylpyrrole (170 mg, 1.8 mmol) and N-ethylcarbazolyl-4-carbaldehyde (200 mg, 0.89 mmol) were dissolved in dry DCM (100 mL) and this reaction mixture was stirred for 50 min, at room temperature, after adding a drop of TFA. Subsequently, a solution of DDQ (406 mg, 1.8 mmol) dissolved in dry DCM (100 mL) was added and the stirring time was extended for another 50 min. Then, triethylamine (2 mL, 15 mmol) was added and, after 15 min, we added BF3.OEt2 (3 mL, 25 mmol) to the reaction mixture, which was further stirred for 30 min at room temperature. The crude product obtained after the evaporation of the solvent under reduced pressure was purified by dry flash chromatography using a petroleum ether/AcOEt (4:1) mixture as eluent. The pure BODIPY derivative 1 (Figure 1) was obtained as a dark red solid (65 mg, 17%).
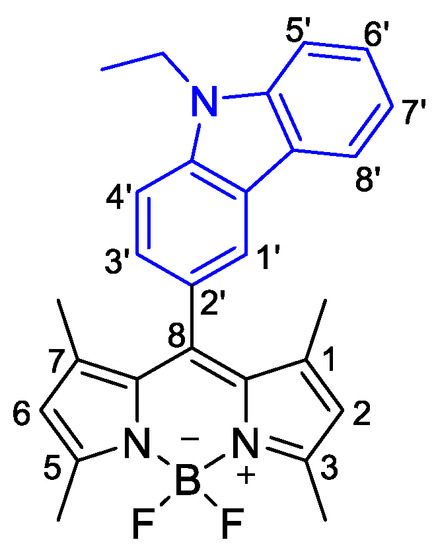
Figure 1.
Structure of BODIPY derivative 1.
1H RMN (400 MHz, CDCl3): δ = 1.27 (s, 6H, CH3-1 and CH3-7), 1.51 (t, 3H, N(CH2CH3)), 2.59 (s, 6H, CH3-3 and CH3-5), 4.45 (q, 2H, N(CH2CH3)), 5.99 (s, 2H, H-2 and H-6), 7.26–7.30 (m, 1H, H6’ or H7’), 7.30 (dd, J = 1.6 and 8.4 Hz, 1H, H3’), 7.48–7.56 (m, 3H, H8’, H4’, H6’ or H7’ ), 8.01 (d, J = 7.6 Hz, 1H, H1’), 8.08 (dd, J = 0.8 and 1.6 Hz, 1H, H5’), ppm.
MS (ESI) m/z (%): 443 ([M+2]+•, 33), 442 ([M+1]+•, 100), 441 ([M]+•, 29), 394 (54), 291 (3), 239 (8), 102 (6); HRMS (ESI) m/z: [M+1]+• calcd for C27H27BF2N3, 442.2261; found 442.2263.
2.3. Photophysical Characterization of BODIPY Derivative 1
The photophysical characterization of BODIPY derivative 1 in acetonitrile (1 × 10−5 M) was carried out using quartz cells. A 1 × 10−5 M Rhodamine 6G (ΦF = 0.95) solution in ethanol was used as the fluorescence standard [13]. The compound and the standard were excited at the maximum absorption wavelength of BODIPY 1. After plotting the fluorescence spectra of compound 1 and Rhodamine 6G, the area under the curve was determined and the relative quantum fluorescence yield of BODIPY 1 was calculated through Equation (1).
As and Acomp, Fs and Fcomp, and ns and ncomp correspond to the absorbances, areas under the fluorescence curve, and refractive index value of the solvent of the standard and compound 1, respectively.
2.4. Chemosensing Studies of BODIPY Derivative 1
The evaluation of BODIPY derivative 1 as an optical chemosensor was carried out in the presence of several anions (H2PO4-, HSO4-, CN-, F-, I-, NO3-, CH3COO-, Br-, ClO4-, BzO-). For this study, solutions of compound 1 (1 × 10−5 M) and the various anions (1 × 10−2 M) were prepared in acetonitrile, and the preliminary study was carried out by the addition of 50 equivalents of each anion to the BODIPY derivative 1 solution. Solutions were visualized under natural light and in a UV-chamber under a lamp at 365 nm.
3. Results and Discussion
3.1. Synthesis of BODPY Derivative 1
Carbazolyl-BODIPY 1 was synthesized in two reactional steps at room temperature. The first step consisted in the condensation reaction of 2,4-dimethylpyrrole and N-ethylcarbazolyl-4-carbaldehyde in dichloromethane (DCM), in the presence of trifluoroacetic acid (TFA) as catalyst. The second reactional step involved oxidation of the dipyrromethane to dipyrromethene, through addition of a solution of 2,3-dichloro-5,6-diciano-p-benzoquinone (DDQ), followed by a complexation reaction with BF3OEt2 in presence of triethylamine (Scheme 1). Finally, the purification of the crude product by dry flash chromatography, using petroleum ether/ethyl acetate (4:1) as eluent, gave the pure BODIPY derivative 1 as a dark red solid at a 17% yield.
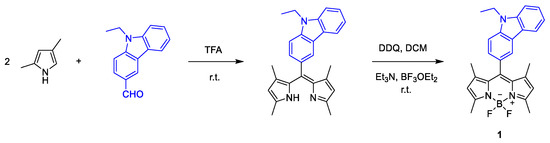
Scheme 1.
Synthesis of BODIPY derivative 1.
Through the 1H NMR spectrum, it was possible to confirm the presence of the carbazolyl group in the meso position of the BODIPY core, with the characteristic proton signals appearing in the aromatic zone of the spectrum. Additionally, the data obtained by mass spectrometry is in agreement with the expected structure.
3.2. Photophysical Characterization of BODIPY Derivative 1
The photophysical characterization of BODIPY derivative 1 was investigated in acetonitrile solution. The compound showed an intense absorption band (log ε = 4.9) at 496 nm. Upon excitation at 480 nm, the compound exhibited an emission band at 507 nm (Figure 2). The relative fluorescence quantum yield, determined by using Rhodamine 6G in ethanol as fluorescence standard (ΦF = 0.95), was found to be low (ΦF = 0.019).
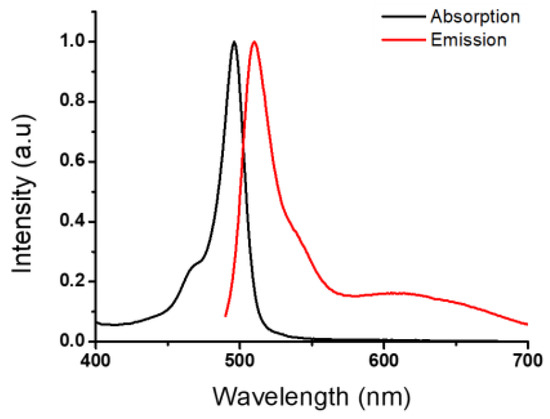
Figure 2.
Normalized absorption and fluorescence spectra of BODIPY 1 in acetonitrile (λmax = 480 nm).
3.3. Chemosensing Studies of BODIPY Derivative 1
Preliminary evaluation of carbazolyl-BODIPY 1 as an optical chemosensor was performed in the presence of several anions in acetonitrile solution. The study was carried out by addition of 50 equivalents of each anion to the compound’s solution and, as can be seen in Figure 3, there was a selective increase in the fluorescence of the compound upon addition of F-.
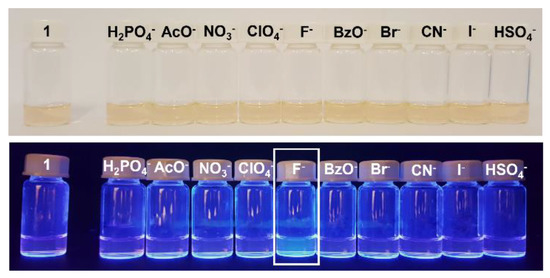
Figure 3.
BODIPY derivative 1 solutions in the presence of various anions, in acetonitrile, under natural light (above) and UV radiation at λmax = 365 nm (below).
4. Conclusions
The BODIPY derivative 1 functionalized at the meso position with a carbazolyl group was synthesized through a condensation reaction between 2,4-dimethylpyrrole and N-ethylcarbazolyl-4-carbaldehyde, at a 17% yield. This compound was characterized by the usual spectroscopic techniques and the photophysical properties were also determined. Additionally, the preliminary chemosensing study of BODIPY derivative 1 shows the selective recognition of F- among several anions, through a perceptible enhancement of the fluorescence of the solution of the compound. Therefore, BODIPY 1 could be a potential selective fluorimetric chemosensor for the detection of fluoride.
Author Contributions
Conceptualization, M.M.M.R.; methodology, M.M.M.R. and S.P.G.C.; validation, M.M.M.R. and S.P.G.C.; formal analysis, S.C.S.P. and R.C.R.G. and M.M.M.R. and S.P.G.C.; investigation, S.C.S.P. and R.C.R.G.; resources, M.M.M.R. and S.P.G.C.; writing—original draft preparation, S.C.S.P. and R.C.R.G.; writing—review and editing, S.C.S.P., R.C.R.G. and M.M.M.R. and S.P.G.C.; supervision, M.M.M.R. and S.P.G.C.; project administration, M.M.M.R.; funding acquisition, M.M.M.R. and S.P.G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Foundation for Science and Technology (FCT) via financial support awarded to CQ/UM (UID/QUI/00686/2020) and project PTDC/QUI-COL/28052/2017. Thanks are also due to Fundação para a Ciência e Tecnologia (Portugal) for financial support given to the Portuguese NMR Network (PTNMR, Bruker Avance III 400-Univ. Minho).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Jiao, Y.; Zhu, B.; Chen, J.; Duan, X. Fluorescent sensing of fluoride in cellular system. Theranostics 2015, 5, 173–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ding, H.; Ran, X.; Tang, H.; Cao, D. Recent progress on reaction-based BODIPY probes for anion detection. Dyes Pigm. 2020, 172, 107857. [Google Scholar] [CrossRef]
- Zlatić, K.; Ayouchia, H.B.E.; Anane, H.; Mihaljević, B.; Basarić, N.; Rohand, T. Spectroscopic and photophysical properties of mono- and dithiosubstituted BODIPY dyes. J. Photochem. Photobiol. A Chem. 2020, 388, 112206. [Google Scholar] [CrossRef]
- Lakshmi, V.; Sharma, R.; Ravikanth, M. Functionalized boron-dipyrromethenes and their applications. Rep. Org. Chem. 2016, 6, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Suganya, S.; Naha, S.; Velmathi, S. A critical review on colorimetric and fluorescent probes for the sensing of analytes via relay recognition from the year 2012–17. ChemistrySelect 2018, 3, 7231–7268. [Google Scholar] [CrossRef]
- Gonçalves, R.; Pina, J.; Costa, S.P.G.; Raposo, M.M.M. Synthesis and characterization of aryl-substituted BODIPY dyes displaying distinct solvatochromic singlet oxygen photosensitization efficiencies. Dyes Pigm. 2021, 196, 109784. [Google Scholar] [CrossRef]
- Presti, M.L.; Martínez-Máñez, R.; Ros-Lis, J.V.; Batista, R.M.F.; Costa, S.P.G.; Raposo, M.M.M.; Sancenón, F. A dual channel sulphur-containing macrocycle functionalised BODIPY probe for the detection of Hg(II) in mixed aqueous solution. New J. Chem. 2018, 42, 7863–7868. [Google Scholar] [CrossRef] [Green Version]
- Collado, D.; Casado, J.; González, S.R.; Navarrete, J.T.L.; Suau, R.; Perez-Inestrosa, E.; Pappenfus, T.M.; Raposo, M.M.M. Enhanced functionality for donor-acceptor oligothiophenes by means of inclusion of BODIPY: Synthesis, electrochemistry, photophysics and model chemistry. Chem. Eur. J. 2011, 17, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.C.R.; Pinto, S.C.S.; Costa, S.P.G.; Raposo, M.M.M. Synthesis, characterization and evaluation of a novel BODIPY derivative as a colorimetric chemosensor for Fe3+ recognition. Proceedings 2019, 41, 40. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, R.; Nogueira, M.; Costa, S.P.G.; Raposo, M.M.M. BODIPY derivatives: Synthesis and evaluation of their optical properties. Proceedings 2019, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, R.; Nogueira, M.; Costa, S.P.G.; Raposo, M.M.M. Functionalized BODIPY derivatives as potential fluorescent labels. Proceedings 2019, 9, 36. [Google Scholar] [CrossRef] [Green Version]
- Pinto, S.C.S.; Gonçalves, R.C.R.; Costa, S.P.G.; Raposo, M.M.M. Synthesis and characterization of a meso-anthracene-BODIPY derivative for colorimetric recognition of Cu2+ and Fe3+. Chem. Proc. 2020, 3, 79. [Google Scholar] [CrossRef]
- Demas, J.N.; Crosby, G.A. The measurement of photoluminescence quantum yields. A Review. J. Phys. Chem. 1971, 75, 991–1024. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).