Design, Synthesis and Bioactivity of Benzimidazole–2–Carbamates as Soil–Borne Anti–Fungal Agents †,‡
Abstract
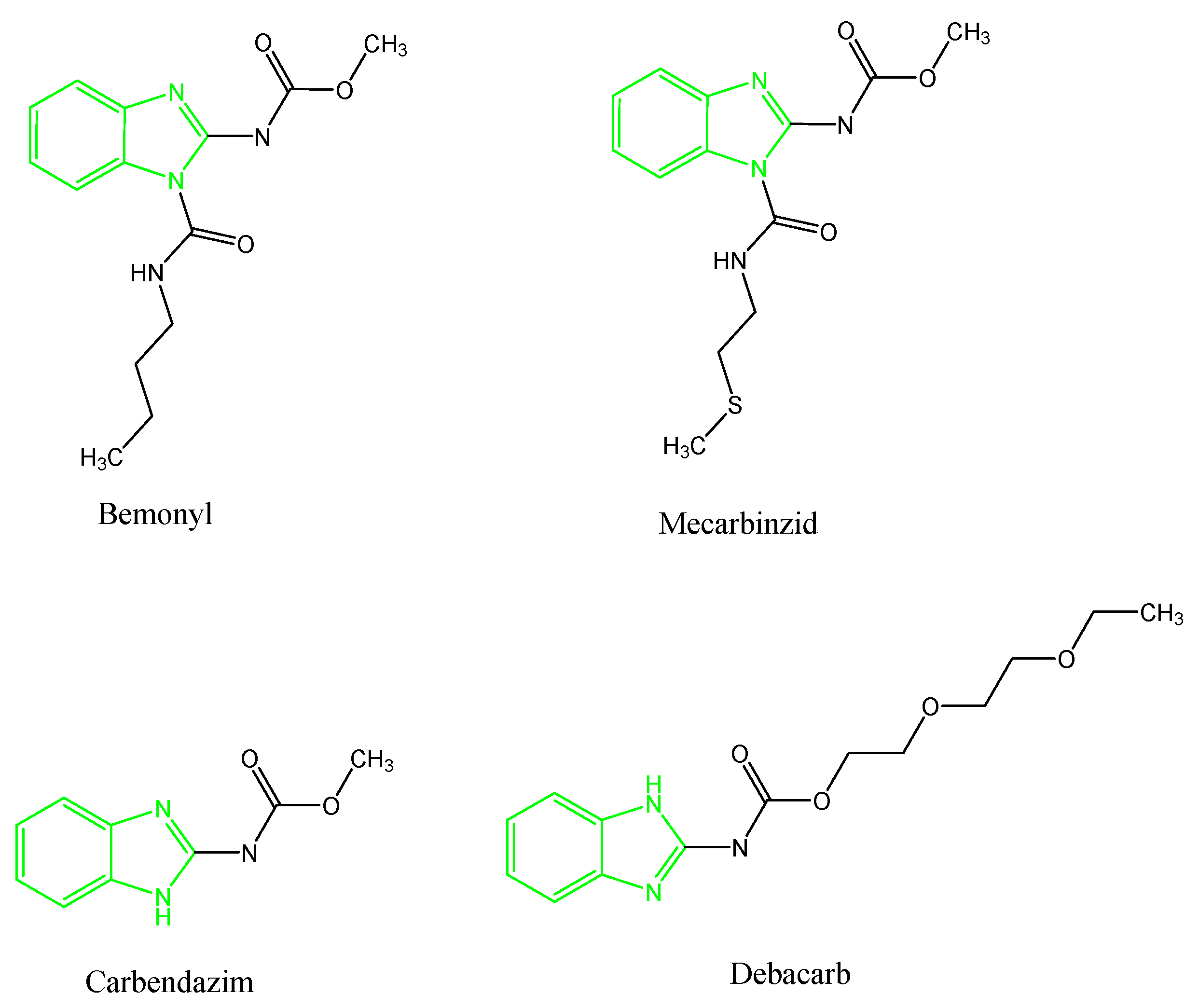
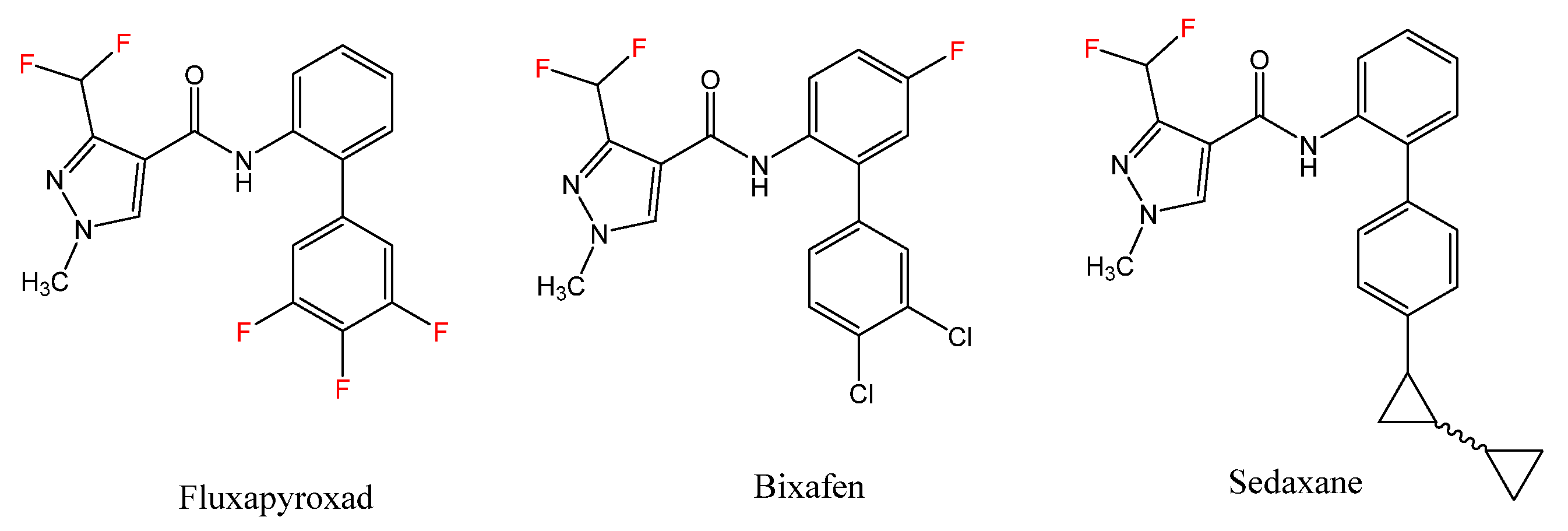
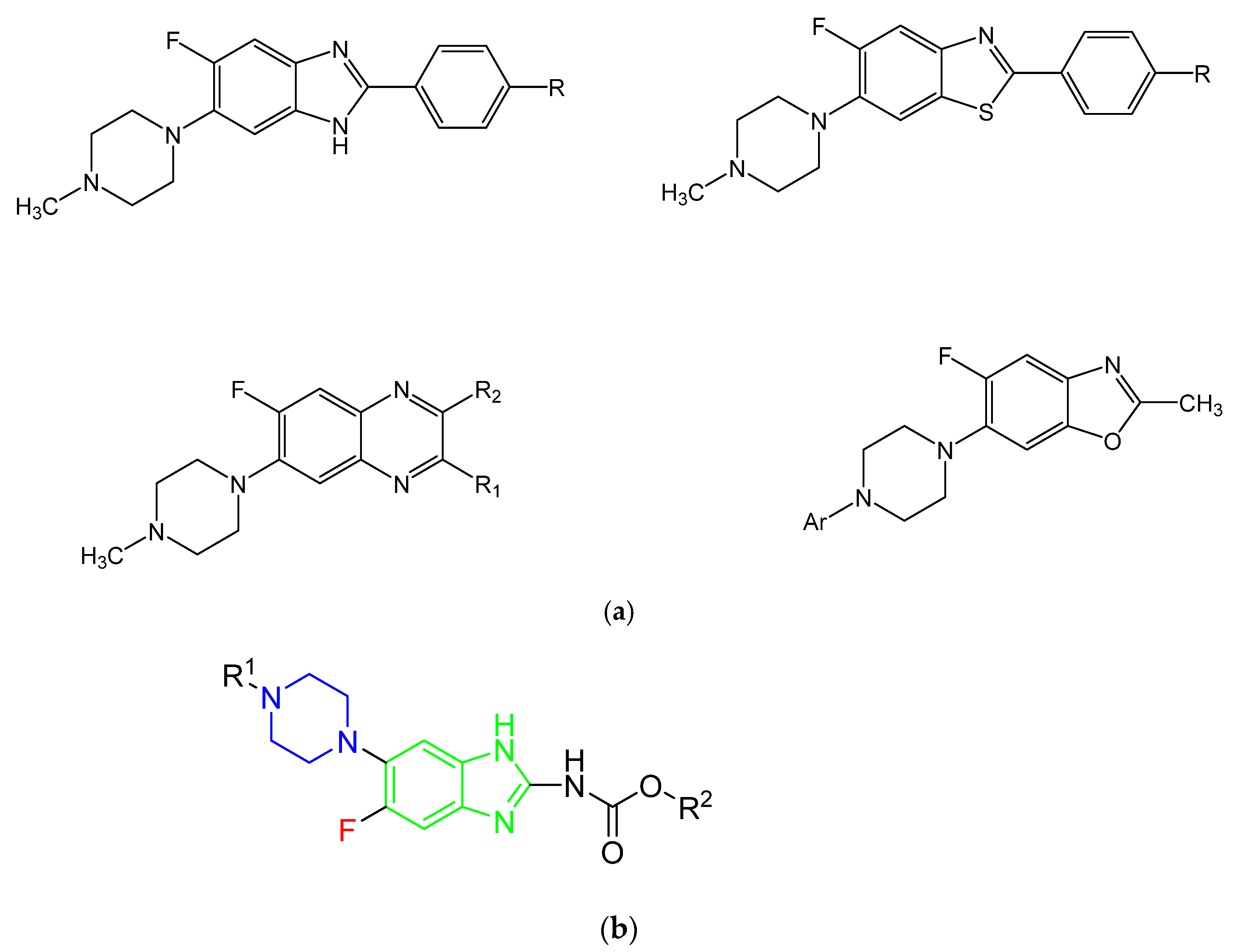
1. Introduction
- To synthesize a novel class of 2-carbamate benzimidazoles.
- To investigate the efficacy of the new fungicide formulations in suppressing the growth of the most common soil-borne pathogens.
2. Results and Discussion
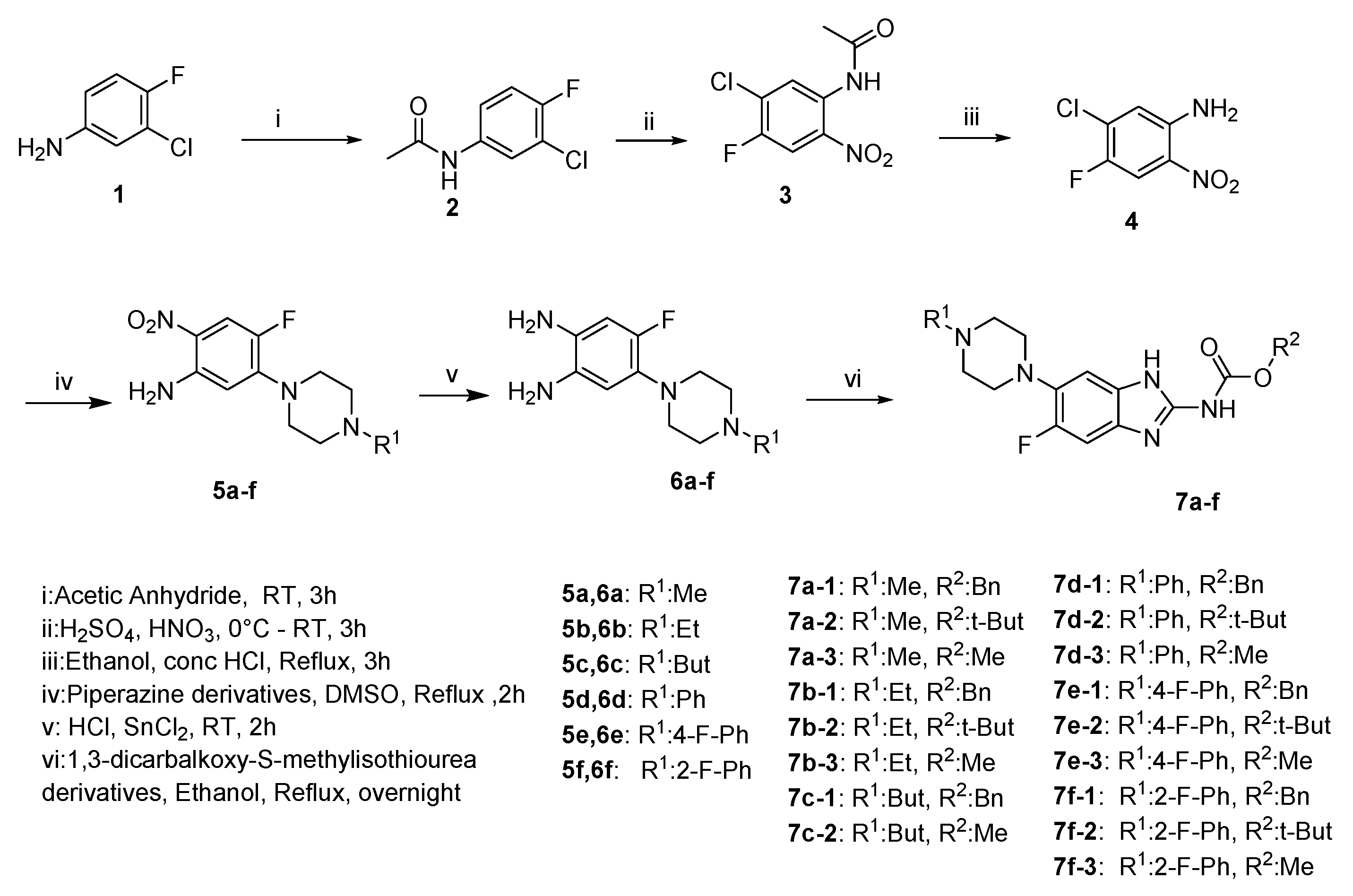
2.1. Chemistry
2.2. Biologic Activity
3. Conclusions
Acknowledgments
Conflicts of Interest
References
- Keri, R.S.; Hiremathad, A.; Budagumpi, S.; Nagaraja, B.M. Comprehensive Review in Current Developments of Benzimidazole-Based Medicinal Chemistry. Chem. Biol. Drug Des. 2015, 86, 19–65. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Xu, J.; Zhu, Y.; Duan, Y.; Zhou, M. Mechanism of Action of the Benzimidazole Fungicide on Fusarium graminearum: Interfering with Polymerization of Monomeric Tubulin But Not Polymerized Microtubule. Phytopathology 2016, 106, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, W.; Khan, M.F.; Verma, G.; Shaquiquzzaman, M.; Rizvi, M.A.; Mehdi, S.H.; Akhter, M.; Alam, M.M. Therapeutic evolution of benzimidazole derivatives in the last quinquennial period. Eur. J. Med. Chem. 2017, 126, 705–753. [Google Scholar] [CrossRef] [PubMed]
- Keri, R.S.; Rajappa, C.K.; Patil, S.A.; Nagaraja, B.M. Benzimidazole-core as an antimycobacterial agent. Pharmacol. Rep. 2016, 68, 1254–1265. [Google Scholar] [CrossRef]
- Gaba, M.; Singh, S.; Mohan, C. Benzimidazole: An emerging scaffold for analgesic and anti-inflammatory agents. Eur. J. Med. Chem. 2014, 76, 494–505. [Google Scholar] [CrossRef] [PubMed]
- Błaszczak-Świątkiewicz, K.; Olszewska, P.; Mikiciuk-Olasik, E. Biological approach of anticancer activity of new benzimidazole derivatives. Pharmacol. Rep. 2014, 66, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Ajani, O.O.; Aderohunmu, D.V.; Ikpo, C.O.; Adedapo, A.E.; Olanrewaju, I.O. Functionalized Benzimidazole Scaffolds: Privileged Heterocycle for Drug Design in Therapeutic Medicine. Arch. Pharm. 2016, 349, 475–506. [Google Scholar] [CrossRef]
- Bansal, Y.; Silakari, O. The therapeutic journey of benzimidazoles: A review. Bioorg. Med. Chem. 2012, 20, 6208–6236. [Google Scholar] [CrossRef]
- Lucas, G.B.; Campbell, C.L.; Lucas, L.T. Diseases Caused by Soilborne Fungi. In Introduction to Plant Diseases: Identification and Management; Springer: Boston, MA, USA, 1992; pp. 162–191. [Google Scholar]
- Yu, X.; Teng, P.; Zhang, Y.-L.; Xu, Z.-J.; Zhang, M.-Z.; Zhang, W.-H. Design, synthesis and antifungal activity evaluation of coumarin-3-carboxamide derivatives. Fitoterapia 2018, 127, 387–395. [Google Scholar] [CrossRef]
- Shivarama Holla, B.; Sooryanarayana Rao, B.; Sarojini, B.K.; Akberali, P.M.; Suchetha Kumari, N. Synthesis and studies on some new fluorine containing triazolothiadiazines as possible antibacterial, antifungal and anticancer agents. Eur. J. Med. Chem. 2006, 41, 657–663. [Google Scholar] [CrossRef]
- Wei, P.; Liu, Y.; Li, W.; Qian, Y.; Nie, Y.; Kim, D.; Wang, M. Metabolic and Dynamic Profiling for Risk Assessment of Fluopyram, a Typical Phenylamide Fungicide Widely Applied in Vegetable Ecosystem. Sci. Rep. 2016, 6, 33898. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yang, W.; Liu, H.; Li, M.; Zhou, W.; Xie, J. Crystal structures and antifungal activities of fluorine-containing thioureido complexes with nickel(II). Molecules 2013, 18, 15737–15749. [Google Scholar] [CrossRef]
- Suryavanshi, H.; Rathore, M. Synthesis and biological activities of piperazine derivatives as antimicrobial and antifungal agents. Org. Commun. 2017, 10, 228–238. [Google Scholar] [CrossRef]
- Lv, H.-S.; Wang, L.-Y.; Ding, X.-L.; Wang, X.-H.; Zhao, B.-X.; Zuo, H. Synthesis and Antifungal Activity of Novel (1-Arylmethyl-3-Aryl-1H-Pyrazol-5-yl)(4-Arylpiperazin-1-yl)Methanone Derivatives. J. Chem. Res. 2013, 37, 473–475. [Google Scholar] [CrossRef]
- Mohsen, U. Synthesis and Antimicrobial Activity of Some Piperazine Dithiocarbamate Derivatives. Turk. J. Pharm. Sci. 2014, 11, 347–354. [Google Scholar]
- Nishat, N.; Haq, M.M.; Ahamad, T.; Kumar, V. Synthesis, spectral and antimicrobial studies of a novel macrocyclic ligand containing a piperazine moiety and its binuclear metal complexes. J. Coord. Chem. 2007, 60, 85–96. [Google Scholar] [CrossRef]
- Kondoh, O.; Inagaki, Y.; Fukuda, H.; Mizuguchi, E.; Ohya, Y.; Arisawa, M.; Shimma, N.; Aoki, Y.; Sakaitani, M.; Watanabe, T. Piperazine Propanol Derivative as a Novel Antifungal Targeting 1,3-β-D-glucan Synthase. Biol. Pharm. Bull. 2005, 28, 2138–2141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zhang, Y.; Zhan, Y.-Z.; Ma, Y.; Hua, X.-W.; Wei, W.; Zhang, X.; Song, H.-B.; Li, Z.-M.; Wang, B.-L. Synthesis, crystal structure and 3D-QSAR studies of antifungal (bis-)1,2,4-triazole Mannich bases containing furyl and substituted piperazine moieties. Chin. Chem. Lett. 2017, 29, 441–446. [Google Scholar] [CrossRef]
- Kazeeroni, E.A.; Al-Sadi, A.M. 454-Pyrosequencing Reveals Variable Fungal Diversity Across Farming Systems. Front. Plant Sci 2016, 7, 314. [Google Scholar] [CrossRef]
- Al-Sadi, A.M. Epidemiology and Management of Fungal Diseases in Dry Environments. In Innovations in Dryland Agriculture; Farooq, M., Siddique, K.H.M., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 187–209. [Google Scholar]
- Al-Sadi, A.M.; Al-Said, F.A.; Al-Kiyumi, K.S.; Al-Mahrouqi, R.S.; Al-Mahmooli, I.H.; Deadman, M.L. Etiology and characterization of cucumber vine decline in Oman. Crop Prot. 2011, 30, 192–197. [Google Scholar] [CrossRef]
- Al-Mawali, Q.; Al-Sadi, A.; Fa, A.-S.; Deadman, M. Etiology, development and reaction of muskmelon to vine decline under arid conditions of Oman. Phytopathol. Mediterr. 2013, 52, 457–465. [Google Scholar]
- Al-Mawaali, Q.S.; Al-Sadi, A.M.; Khan, A.J.; Al-Hasani, H.D.; Deadman, M.L. Response of cucurbit rootstocks to Pythium aphanidermatum. Crop Prot. 2012, 42, 64–68. [Google Scholar] [CrossRef]
- Hatami, N.; Aminaee, M.M.; Zohdi, H.; Tanideh, T. Damping-off disease in greenhouse cucumber in Iran. Arch. Phytopathol. Plant Prot. 2013, 46, 796–802. [Google Scholar] [CrossRef]
- Huang, X.; Liu, L.; Wen, T.; Zhang, J.; Shen, Q.; Cai, Z. Reductive soil disinfestations combined or not with Trichoderma for the treatment of a degraded and Rhizoctonia solani infested greenhouse soil. Sci. Hortic. 2016, 206, 51–61. [Google Scholar] [CrossRef]
- Abbasi, P.A.; Renderos, W.; Fillmore, S. Soil incorporation of buckwheat as a pre-plant amendment provides control of Rhizoctonia damping-off and root rot of radish and Pythium damping-off and root rot of cucumber. Can. J. Plant Pathol. 2019, 41, 24–34. [Google Scholar] [CrossRef]
- Al-Fadhal, F.A.; Al-Abedy, A.N.; Alkhafije, D.A. Isolation and molecular identification of Rhizoctonia solani and Fusarium solani isolated from cucumber (Cucumis sativus L.) and their control feasibility by Pseudomonas fluorescens and Bacillus subtilis. Egypt. J. Biol. Pest. Control. 2019, 29, 47. [Google Scholar] [CrossRef]
- Owen, W.; Jackson, B.; Whipker, B.E.; Fonteno, W.; Benson, M.D. Assessing the severity of damping-off caused by Pythium ultimum and Rhizoctonia solani in peat-based greenhouse substrates amended with pine wood chip aggregates. Acta Hortic. 2019, 1266, 27–34. [Google Scholar] [CrossRef]
- Philosoph, A.; Dombrovsky, A.; Elad, Y.; Koren, A.; Frenkel, O. Insight Into Late Wilting Disease of Cucumber Demonstrates the Complexity of the Phenomenon in Fluctuating Environments. Plant Dis. 2019, 103, 2877–2883. [Google Scholar] [CrossRef]
- Ravnskov, S.; Cabral, C.; Larsen, J. Mycorrhiza induced tolerance in Cucumis sativus against root rot caused by Pythium ultimum depends on fungal species in the arbuscular mycorrhizal symbiosis. Biol. Control. 2020, 141, 104133. [Google Scholar] [CrossRef]
- Al-Sadi, A.M.; Al-Masoudi, R.S.; Al-Habsi, N.; Al-Said, F.A.; Al-Rawahy, S.A.; Ahmed, M.; Deadman, M.L. Effect of salinity on Pythium damping-off of cucumber and on the tolerance of Pythium aphanidermatum. Plant Pathol. 2010, 59, 112–120. [Google Scholar] [CrossRef]
- Al-Sadi, A.M.; Al-Said, F.A.; Al-Jabri, A.H.; Al-Mahmooli, I.H.; Al-Hinai, A.H.; de Cock, A.W.A.M. Occurrence and characterization of fungi and oomycetes transmitted via potting mixtures and organic manures. Crop Prot. 2011, 30, 38–44. [Google Scholar] [CrossRef]
- De Corato, U.; Patruno, L.; Avella, N.; Lacolla, G.; Cucci, G. Composts from green sources show an increased suppressiveness to soilborne plant pathogenic fungi: Relationships between physicochemical properties, disease suppression, and the microbiome. Crop Prot. 2019, 124, 104870. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Graber, E.R.; Elad, Y.; Frenkel, O. Biochar as a management tool for soilborne diseases affecting early stage nursery seedling production. Crop Prot. 2019, 120, 34–42. [Google Scholar] [CrossRef]
- Wang, H.; Ding, J.; Liu, S.; Bai, X.; Xue, L. Different carbonic supplements induced changes of microflora in two types of compost teas and biocontrol efficiency against Pythium aphanidermatum. Biocontrol Sci. Technol. 2019, 29, 924–939. [Google Scholar] [CrossRef]
- You, X.; Kimura, N.; Okura, T.; Murakami, S.; Okano, R.; Shimogami, Y.; Matsumura, A.; Tokumoto, H.; Ogata, Y.; Tojo, M. Suppressive Effects of Vermicomposted-Bamboo Powder on Cucumber Damping-Off. Jpn. Agric. Res. Q. 2019, 53, 13–19. [Google Scholar] [CrossRef]
- Al-Sa’di, A.M.; Drenth, A.; Deadman, M.L.; Al-Said, F.A.; Khan, I.; Aitken, E.A.B. Association of a second phase of mortality in cucumber seedlings with a rapid rate of metalaxyl biodegradation in greenhouse soils. Crop Prot. 2008, 27, 1110–1117. [Google Scholar] [CrossRef]
- Al-Sadi, A.M. Efficacy of mefenoxam is affected by a lag period between application and inactivation of Pythium species. Phytopathol. Mediterr. 2012, 51, 292–297. [Google Scholar]
- Al-Sadi, A.M.; Al-Masoodi, R.S.; Al-Ismaili, M.; Al-Mahmooli, I.H. Population Structure and Development of Resistance to Hymexazol Among Fusarium solani Populations from Date Palm, Citrus and Cucumber. J. Phytopathol. 2015, 163, 947–955. [Google Scholar] [CrossRef]
- Al-Balushi, Z.; Agrama, H.; Al-Mahmooli, I.; Maharachchikumbura, S.; Al-Sadi, A. Development of Resistance to Hymexazol Among Pythium Species in Cucumber Greenhouses in Oman. Plant Dis. 2018, 102, 202–208. [Google Scholar] [CrossRef]
- Halo, B.A.; Al-Yahyai, R.A.; Al-Sadi, A.M. Aspergillus terreus Inhibits Growth and Induces Morphological Abnormalities in Pythium aphanidermatum and Suppresses Pythium-Induced Damping-Off of Cucumber. Front. Microbiol 2018, 9, 95. [Google Scholar] [CrossRef]
- Al-Daghari, D.S.S.; Al-Abri, S.A.; Al-Mahmooli, I.H.; Al-Sadi, A.M.; Velazhahan, R. Efficacy of native antagonistic rhizobacteria in the biological control of Pythium aphanidermatum-induced damping-off of cucumber in Oman. J. Plant Pathol. 2020, 102, 305–310. [Google Scholar] [CrossRef]
- Al-Shibli, H.; Dobretsov, S.; Al-Nabhani, A.; Maharachchikumbura, S.S.N.; Rethinasamy, V.; Al-Sadi, A.M. Aspergillus terreus obtained from mangrove exhibits antagonistic activities against Pythium aphanidermatum-induced damping-off of cucumber. PeerJ 2019, 7, e7884. [Google Scholar] [CrossRef] [PubMed]
- Halo, B.A.; Al-Yahyai, R.A.; Maharachchikumbura, S.S.N.; Al-Sadi, A.M. Talaromyces variabilis interferes with Pythium aphanidermatum growth and suppresses Pythium-induced damping-off of cucumbers and tomatoes. Sci. Rep. 2019, 9, 11255. [Google Scholar] [CrossRef] [PubMed]
- Kazerooni, E.; Velazhahan, R.; Al-Sadi, A. Talaromyces pinophilus inhibits Pythium and Rhizoctonia-induced damping-off of cucumber. J. Plant Pathol. 2018, 101, 377–383. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al–Harthy, T.; Al-Sadi, A.M.; Zoghaib, W.; Moghadam, E.S.; Stoll, R.; Abdel-Jalil, R. Design, Synthesis and Bioactivity of Benzimidazole–2–Carbamates as Soil–Borne Anti–Fungal Agents †,‡. Chem. Proc. 2021, 3, 64. https://doi.org/10.3390/ecsoc-24-08093
Al–Harthy T, Al-Sadi AM, Zoghaib W, Moghadam ES, Stoll R, Abdel-Jalil R. Design, Synthesis and Bioactivity of Benzimidazole–2–Carbamates as Soil–Borne Anti–Fungal Agents †,‡. Chemistry Proceedings. 2021; 3(1):64. https://doi.org/10.3390/ecsoc-24-08093
Chicago/Turabian StyleAl–Harthy, Thuraya, Abdullah M. Al-Sadi, Wajdi Zoghaib, Ebrahim Saeedian Moghadam, Raphael Stoll, and Raid Abdel-Jalil. 2021. "Design, Synthesis and Bioactivity of Benzimidazole–2–Carbamates as Soil–Borne Anti–Fungal Agents †,‡" Chemistry Proceedings 3, no. 1: 64. https://doi.org/10.3390/ecsoc-24-08093
APA StyleAl–Harthy, T., Al-Sadi, A. M., Zoghaib, W., Moghadam, E. S., Stoll, R., & Abdel-Jalil, R. (2021). Design, Synthesis and Bioactivity of Benzimidazole–2–Carbamates as Soil–Borne Anti–Fungal Agents †,‡. Chemistry Proceedings, 3(1), 64. https://doi.org/10.3390/ecsoc-24-08093







