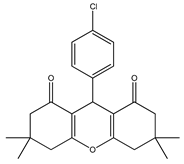
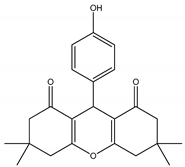
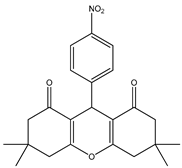
Cu.BTC MOF as a Novel and Efficient Catalyst for the Synthesis of 1,8-Dioxo-octa-hydro Xanthene †
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of Cu.BTC MOF
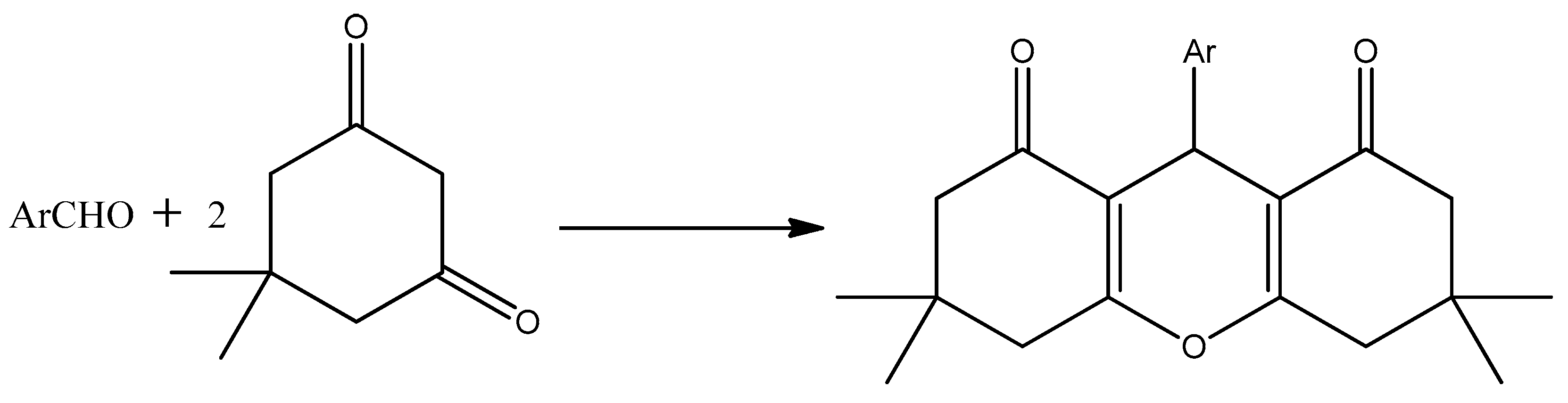
2.3. Synthesis of 1,8-Dioxo-octa-hydro Xanthene Derivatives
3. Characterization
4. Results and Discussion
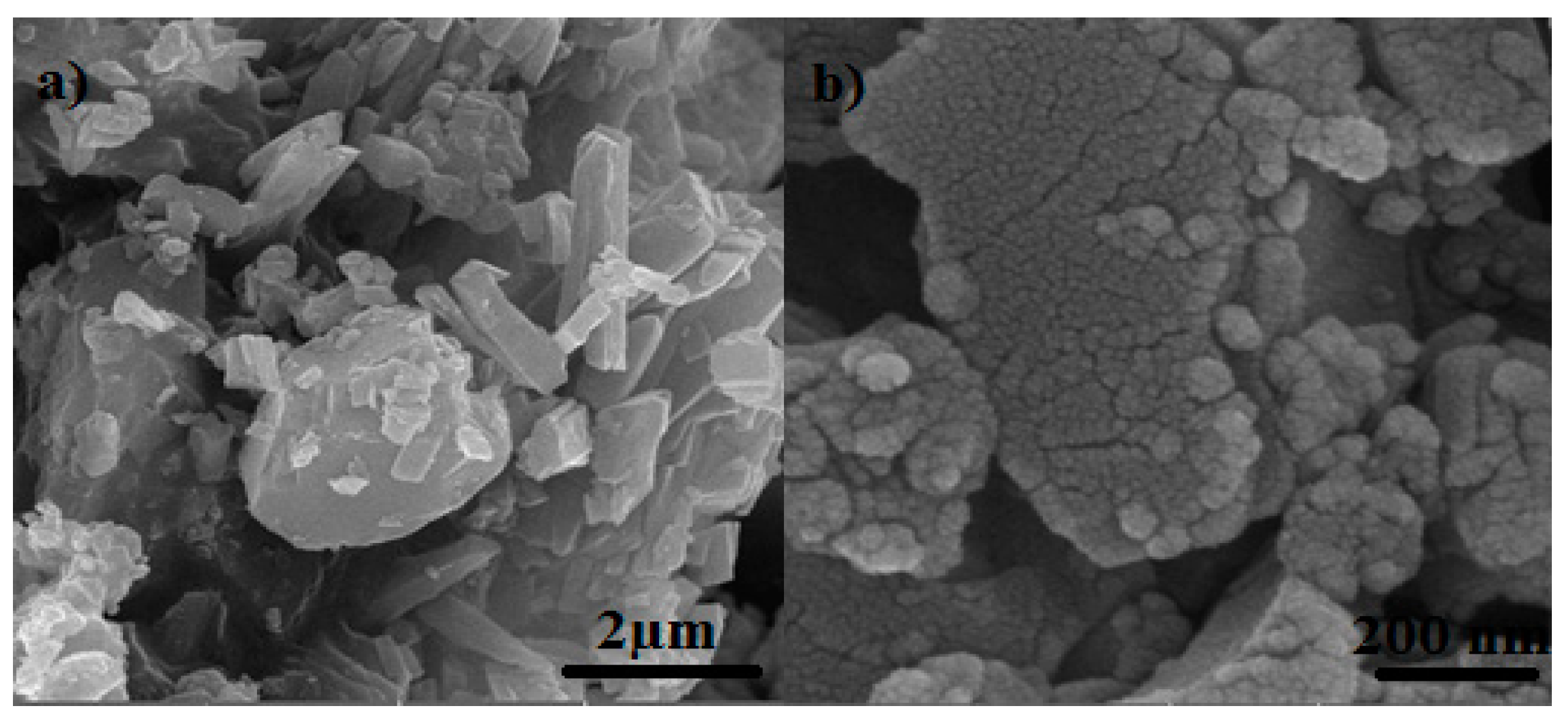
4.1. FE-SEM
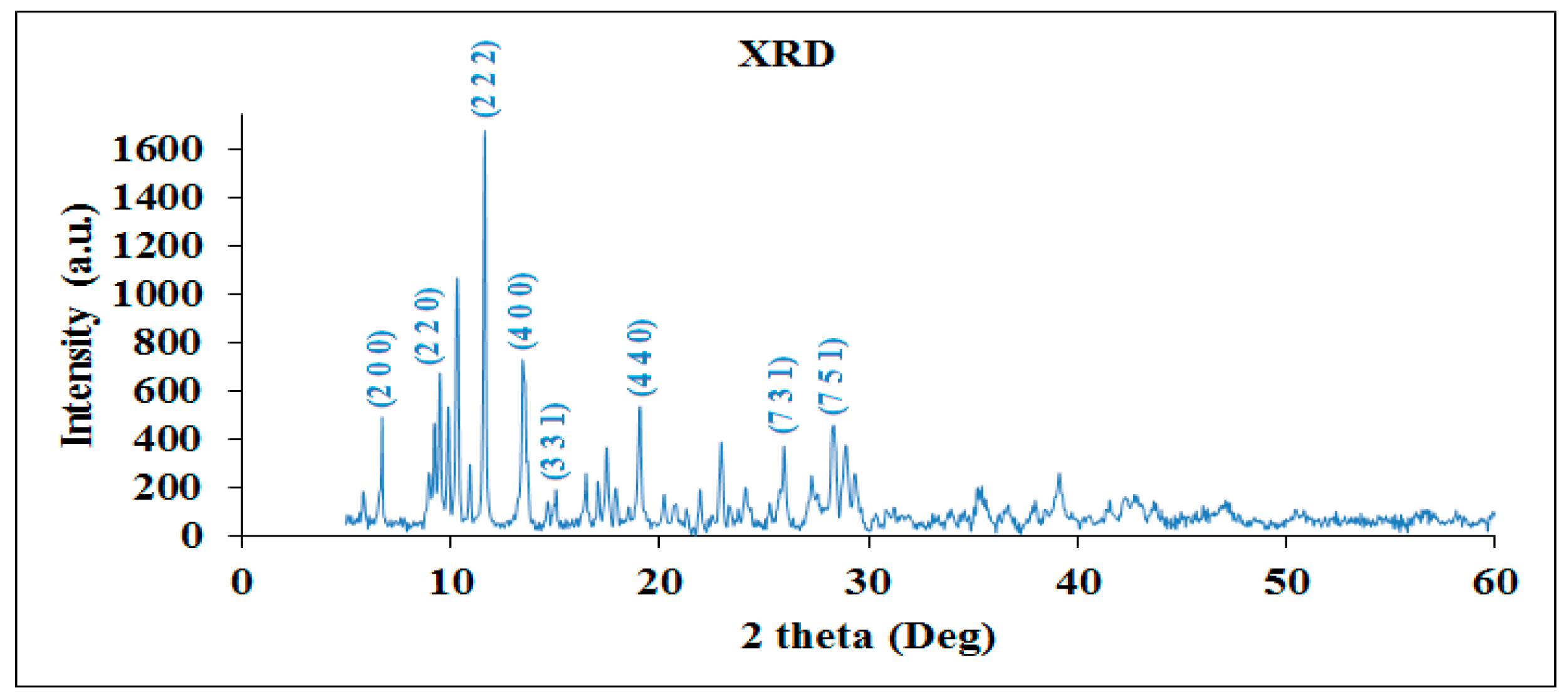
4.2. XRD
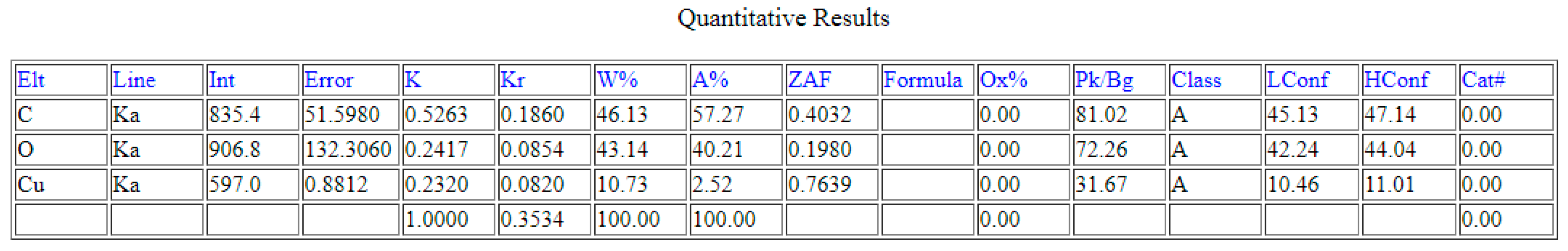
4.3. EDS
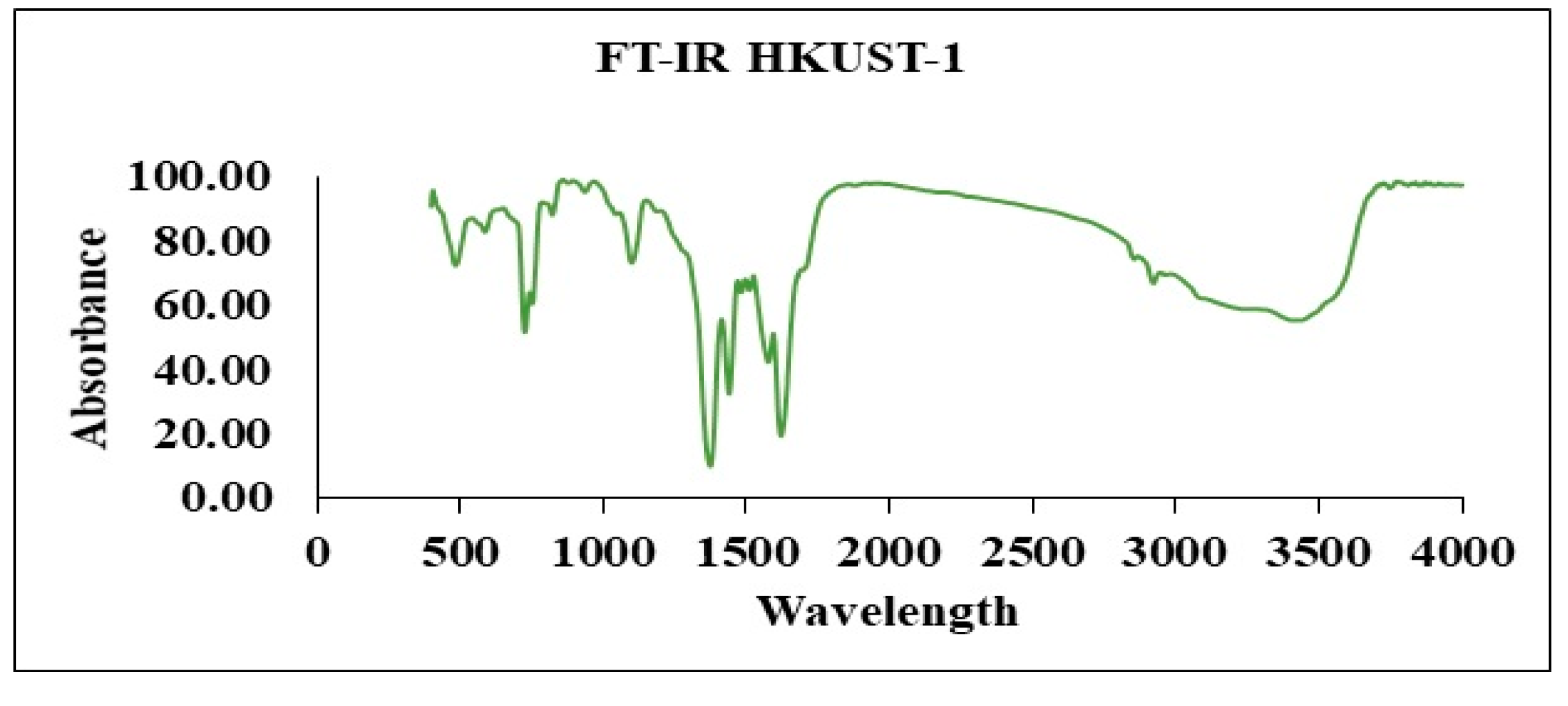
4.4. FT-IR
4.5. BET
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Ganem, B. Strategies for innovation in multicomponent reaction design. Acc. Chem. Res. 2009, 42, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Evdokimov, N.M.; Kireev, A.S.; Yakovenko, A.A.; Antipin, M.Y.; Magedov, I.V.; Kornienko, A. One-step synthesis of heterocyclic privileged medicinal scaffolds by a multicomponent reaction of malononitrile with aldehydes and thiols. J. Org. Chem. 2007, 72, 3443–3453. [Google Scholar] [CrossRef] [PubMed]
- Nile, S.H.; Khobragade, C.N. In vitro anti-inflammatory and xanthine oxidase inhibitory activity of Tephrosia purpurea shoot extract. Nat. Prod. Commun. 2011, 6, 1934578X1100601006. [Google Scholar] [CrossRef]
- Parmar, B.; Patel, P.; Murali, V.; Rachuri, Y.; Kureshy, R.I.; Noor-ul, H.K.; Suresh, E. Efficient heterogeneous catalysis by dual ligand Zn (II)/Cd (II) MOFs for the Knoevenagel condensation reaction: Adaptable synthetic routes, characterization, crystal structures and luminescence studies. Inorg. Chem. Front. 2018, 5, 2630–2640. [Google Scholar] [CrossRef]
- Nobar, S.N. Cu-BTC synthesis, characterization and preparation for adsorption studies. Mater. Chem. Phys. 2018, 213, 343–351. [Google Scholar] [CrossRef]
- Mao, Y.; Cao, W.; Li, J.; Liu, Y.; Ying, Y.; Sun, L.; Peng, X. Enhanced gas separation through well-intergrown MOF membranes: Seed morphology and crystal growth effects. J. Mater. Chem. A 2013, 1, 11711–11716. [Google Scholar] [CrossRef]
- Chen, Y.; Mu, X.; Lester, E.; Wu, T. High efficiency synthesis of HKUST-1 under mild conditions with high BET surface area and CO2 uptake capacity. Prog. Nat. Sci. Mater. Int. 2018, 28, 584–589. [Google Scholar] [CrossRef]
- Lestari, W.W.; Ni’maturrohmah, D.; Arrozi, U.S.F.; Suwarno, H. Mg 2+ Doped into Electro-synthesized HKUST-1 and Their Initial Hydrogen Sorption Properties. Mater. Sci. Eng. 2018, 299, 012031. [Google Scholar]
- Azad, F.N.; Ghaedi, M.; Dashtian, K.; Hajati, S.; Pezeshkpour, V. Ultrasonically assisted hydrothermal synthesis of activated carbon–HKUST-1-MOF hybrid for efficient simultaneous ultrasound-assisted removal of ternary organic dyes and antibacterial investigation: Taguchi optimization. Ultrason. Sonochem. 2016, 31, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Li, F.; Li, L.; Zhang, H.; Wang, X.; Xiao, J.; Xi, H. Hierarchically structured metal–organic frameworks assembled by hydroxy double salt–template synergy with high space–time yields. CrystEngComm 2018, 20, 1057–1064. [Google Scholar] [CrossRef]
- Nasreen, A. Cupric Nitrate Catalyzed Efficient and Facile Synthesis of 1,8-Dioxo-octahydroxanthene Derivatives. Asian J. Chem. 2013, 25, 7535–7538. [Google Scholar] [CrossRef]

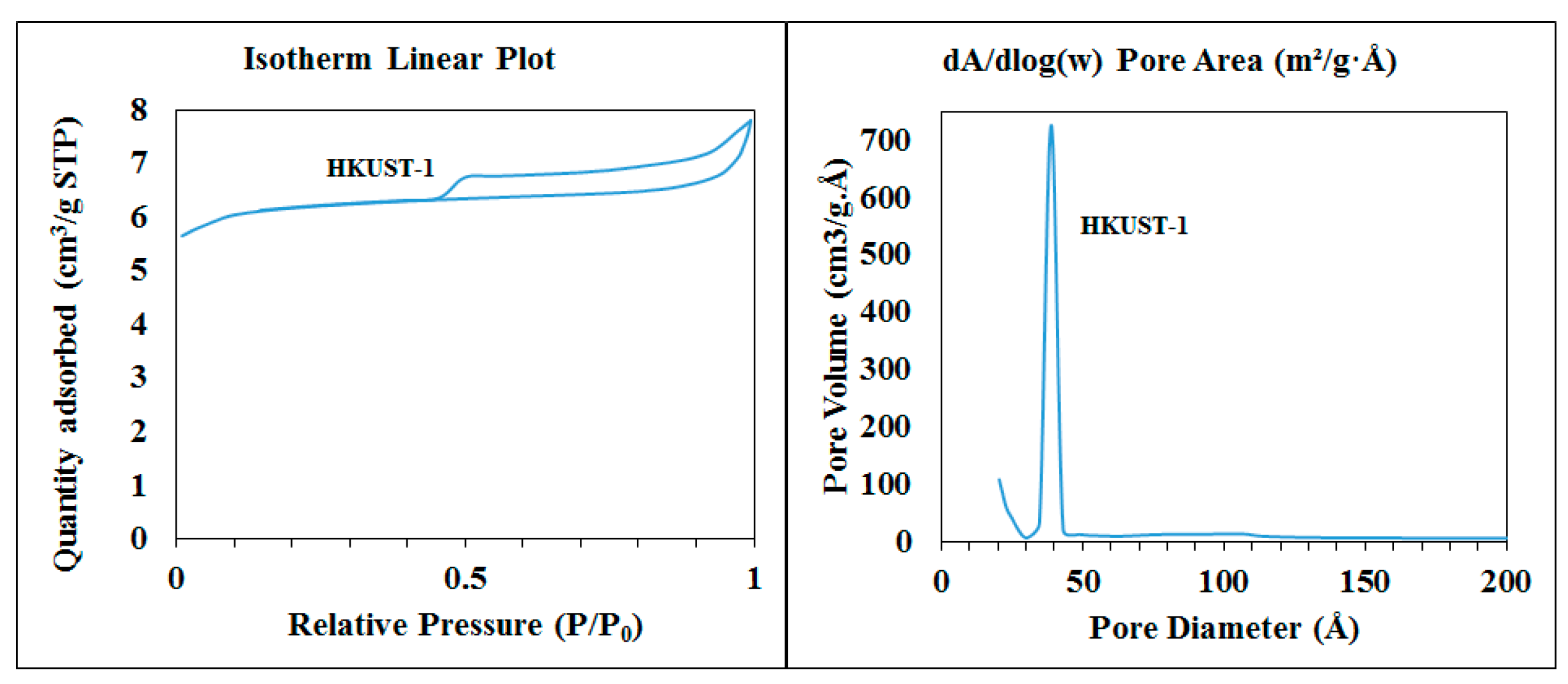
| Sample | BET Surface Area m2/g | Langmuir Surface Area m2/g | BJH Desorption Cumulative Volume of Pores cm3/g | BJH Desorption Average Pore Width Å |
|---|---|---|---|---|
| HKUST-1 | 463.3874 | 613.5577 | 0.080150 | 60.639 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghafuri, H.; Ganjali, F.; Hanifehnejad, P. Cu.BTC MOF as a Novel and Efficient Catalyst for the Synthesis of 1,8-Dioxo-octa-hydro Xanthene. Chem. Proc. 2021, 3, 2. https://doi.org/10.3390/ecsoc-24-08359
Ghafuri H, Ganjali F, Hanifehnejad P. Cu.BTC MOF as a Novel and Efficient Catalyst for the Synthesis of 1,8-Dioxo-octa-hydro Xanthene. Chemistry Proceedings. 2021; 3(1):2. https://doi.org/10.3390/ecsoc-24-08359
Chicago/Turabian StyleGhafuri, Hossein, Fatemeh Ganjali, and Peyman Hanifehnejad. 2021. "Cu.BTC MOF as a Novel and Efficient Catalyst for the Synthesis of 1,8-Dioxo-octa-hydro Xanthene" Chemistry Proceedings 3, no. 1: 2. https://doi.org/10.3390/ecsoc-24-08359
APA StyleGhafuri, H., Ganjali, F., & Hanifehnejad, P. (2021). Cu.BTC MOF as a Novel and Efficient Catalyst for the Synthesis of 1,8-Dioxo-octa-hydro Xanthene. Chemistry Proceedings, 3(1), 2. https://doi.org/10.3390/ecsoc-24-08359