Abstract
3-(Biphenyl)acrylates are prepared in good yield by one-pot Suzuki cross-coupling (Wittig olefination reactions). The central building blocks are 4-formyl- and 3-formylphenylboronic acids and the stabilized (carbomethoxymethylene)triphenylphosphorane. Examples of one-pot Suzuki–double Wittig combinations are also shown.
1. Introduction
One-pot transformations can significantly reduce the time and resources needed for the experimental work-up as compared to carrying out these reactions consecutively. Important for such one-pot transformations is the compatibility of two or more reactions with one set of reaction conditions, including a common solvent system. Carbalkoxymethylenetriphenylphosphoranes (1) as stabilized phosphoranes are reactive enough to undergo Wittig olefination reactions with both aldehydes and ketones but are not sensitive towards water (and oxygen/air) and tolerate a number of solvent systems that include THF, DMSO, DME and CHCl3, aqueous systems [], aqueous/organic solvent mixtures and even solventless conditions []. This makes it possible to employ these phosphoranes to run Wittig olefination reactions in parallel with C-C cross-coupling reactions, such as Suzuki [,], Heck [] or Sonogashira [] reactions in one pot. In the following, we explored the one-pot Wittig olefination–Suzuki cross-coupling reaction utilizing (carbomethoxymethylene)triphenylphosphorane (1a) to substituted 3-(biphenyl)acrylates (2) (Figure 1). This includes combinations where a Suzuki reaction is accompanied by two Wittig olefination reactions and where a hydrolysis as a follow reaction in one pot is incorporated. The work is seen as an expansion of the scope of one-pot Suzuki–Wittig olefination reactions, published by us earlier [].
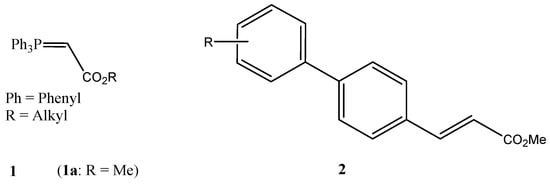
Figure 1.
Carbalkoxymethylenetriphenylphosphoranes as stabilized Wittig reagents (1) and 3-(biphenyl)acrylates as target compounds (2).
2. Materials and Methods
2.1. General Remarks
Melting points were measured on a Stuart SMP 10 melting point apparatus and were uncorrected. Infrared spectra were measured with a Thermo/Nicolet Nexus 470 FT-IR ESP spectrometer and a Perkin Elmer Spectrum Two spectrometer. 1H and 13C NMR spectra were recorded with a Varian 400 NMR spectrometer (1H at 395.7 MHz, 13C at 100.5 MHz). The assignments of the carbon signals were aided by Distortionless Enhancement by Polarisation Transfer (DEPT) 90 and DEPT 135 experiments. The chemical shifts are relative to TMS (solvent CDCl3, unless otherwise noted). Column chromatography, where necessary, was performed on recycled silica gel (S, 0.063 mm–0.1 mm, Riedel de Haen and Merck grade 9385).
2.2. Starting Materials
Triphenylphosphine (6, Aldrich), iodomethane (4a, Sigma-Aldrich), iodoethane (4b, BDH), 1-iodopropane (4c, Alfa Products), 1-iodobutane (4d, Merck-Schuchardt), 4-bromobenzaldehyde (11, Aldrich), 3-bromobenzaldehyde (18, Aldrich), 4-bromobenzoic acid (9b, Merck-Schuchardt), 3-bromobenzoic acid (9a, Merck-Schuchardt), 4-bromophenol (3, Acros), methyl bromoacetate (7, Aldrich), (triphenylphosphoranylidene)acetaldehyde (12, Aldrich), 4-formylphenylboronic acid (14, Aldrich), 3-formylphenylboronic acid (16, Aldrich), 4-nitrophenylboronic acid (25, Aldrich), 3-nitrophenylboronic acid (21, Aldrich), p-tolylboronic acid (23, Aldrich), bis(triphenylphosphine) palladium (II) dichloride (15, Aldrich), potassium hydroxide (KOH, Merck), chloroform (CHCl3-Sigma Aldrich dichloromethane (CH2Cl2, Sigma-Aldrich), 1,2-dimethoxyethane (DME, Sigma-Aldrich) and sodium carbonate (Riedel de Haen) were acquired commercially. Reagents and solvents were used as is. The reactions were run under argon (Air Products; 99.9992%). 4-Alkoxybromobenzenes 5 are prepared from 4-bromophenol (3, KOH, DMSO; then R-I (4), where I = Me, Et, Pr, Bu) (Scheme 1). (Carbomethoxymethylene)triphenylphosphorane (1a) was prepared by the reaction of methyl bromoacetate (7) with triphenylphosphine (6, CHCl3, precipitated upon addition of diethyl ether) with the subsequent reaction of carbomethoxymethyltriphenylphosphonium bromide (8) in a biphasic system of aq. Na2CO3 and CH2Cl2 (Scheme 1). Methyl 4-bromo- and 3-bromobenzoates 10 were prepared from 3-bromo- and 4-bromobenzoic acid 9, respectively (CH3OH, cat. H2SO4) (Scheme 1). 4-Bromocinnamaldehyde (13) was prepared by Wittig reaction of 4-bromobenzaldehyde (11) with (triphenylphosphoranylidene)acetaldehyde (12) (Scheme 1).
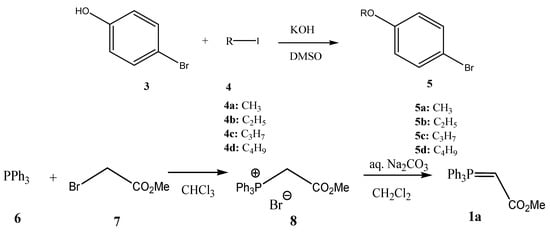
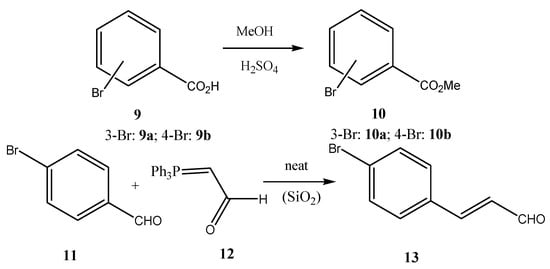
Scheme 1.
Preparation of starting materials.
2.3. Preparation of Methyl 3-[(4′-Ethoxy-1,1′-biphenyl]-4-yl)acrylate (2b)
A reaction mixture of 4-formylphenylboronic acid (14, 300 mg, 2.0 mmol), bromo-4-ethoxybenzene (5b, 402 mg, 2.0 mmol), methoxycarbonylmethylidenetriphenyl-phosphorane (1a, 940 mg, 2.82 mmol), Pd(PPh3)2Cl2 (15, 32 mg, 4.6 × 10−5 mol), and triphenylphosphine (6, PPh3, 32 mg, 1.2 × 10−4 mol) in the biphasic solvent system aq. Na2CO3 (10 mL, 2.32 g Na2CO3 in 10 mL H2O) and 1,2-dimethoxyethane (DME, 10 mL) was stirred at 70 °C for 12 h. Thereafter, the cooled mixture was extracted with chloroform (3 × 20 mL) and water (50 mL). The organic phase was dried over anhydrous MgSO4, filtered, and concentrated in vacuo. The residue was subjected to column chromatography on silica gel (CH2Cl2/CHCl3 5:1) to give 2b (502 mg, 89%) as a glittering colorless solid; IR υ (KBr, cm−1): 2972, 2929, 1723, 1638, 1598, 1312, 1260, 1193, 1170, 1046, 984, 825, 810; δH (400 MHz, CDCl3): 1.44 (3H, t, 3J = 6.8 H), 3.82 (3H, s, OCH3), 4.08 (2H, q, 3J = 6.8 Hz, OCH2), 6.46 (1H, d, 3J = 16.0 Hz), 6.96–6.98 (2H, m), 7.53–7.57 (6H, m), 7.72 (1H, d, 3J = 16.0 Hz); δC (100.5 MHz, CDCl3): 14.8 (CH3), 51.7 (OCH3), 63.5 (OCH2), 114.8 (2C, CH), 117.2 (CH), 127.0 (2C, CH), 128.1 (2C, CH), 128.6 (2C, CH), 132.4 (Cquat), 132.6 (Cquat), 142.7 (Cquat), 144.5 (CH), 159.0 (Cquat), 167.5 (Cquat, CO).
2.4. Preparation of Methyl 3-([4′-Methoxycarbonyl-1,1′-biphenyl]-4-yl)acrylate (2e)
A mixture of methyl 4-bromobenzoate (10b, 430 mg, 2.0 mmol), 4-formylphenylboronic acid (14, 350 mg, 2.33 mmol), methoxycarbonylmethylidenetriphenylphosphorane (1a, 940 mg, 3.0 mmol), bis(triphenylphosphine)palladium (II) dichloride (15, 32 mg, 4.56 × 10−2 mmol) and triphenylphosphine (6, 32 mg, 1.2 × 10−4 mol) in 1,2-dimethoxyethane (DME, 10 mL) and aq. Na2CO3 (2.32 g Na2CO3 in 10 mL H2O) was saturated with argon. Thereafter, the mixture was heated at 82 °C for 12 h. The cooled mixture was added to water (70 mL) and extracted with CHCl3 (2 × 50 mL). The organic phase was separated, dried over anhydrous MgSO4 and evaporated in vacuo. The solid residue was taken up partially in CHCl3 (65 mL). The remaining solid was filtered off, washed with CHCl3 (3 × 20 mL) and air dried to yield the title compound (2e) (410 mg). The filtrate was separated by column chromatography on silica gel (CHCl3/CH2Cl2 1:10) to give further (2e) (136 mg) for a combined yield of 546 mg (91%); IR υ (KBr, cm−1): 3015, 2935, 1725, 1640, 138, 1316, 1289, 1273, 1173, 1114, 985, 830, 777, 740, 697; δH (400 MHz, CDCl3): 3.82 (3H, s, CO2CH3), 3.94 (3H, s, CO2CH3), 6.49 (1H, d, 3J = 16.0 Hz), 7.61 (2H, d, 3J = 8.4 Hz), 7.64 (2H, d, 3J = 8.4 Hz), 7.67 (2H, d, 3J = 8.8 Hz), 7.73 (1H, d, 3J = 16.0 Hz), 8.11 (2H, d, 3J = 8.8 Hz); δC (100.5 MHz, CDCl3): 51.8 (CO2CH3), 52.2 (CO2CH3), 118.2 (CH), 126.9 (2C, CH), 127.7 (2C, CH), 128.7 (2C, CH), 129.3 (Cquat), 130.2 (2C, CH), 134.1 (Cquat), 141.7 (Cquat), 144.1 (CH), 144.5 (Cquat), 166.8 (Cquat), 167.3 (Cquat).
2.5. Preparation of Dimethyl Biphenyl-4,4′-diacrylate (20)—One-Pot Suzuki–Double Wittig Olefination
A mixture of 4-formylphenylboronic acid (14, 300 mg, 2.0 mmol), 4-bromobenzaldehyde (11, 370 mg, 2.0 mmol), methoxycarbonylmethylidenetriphenylphosphorane (1a, 1.87 g, 6.0 mmol), bis(triphenylphosphine)palladium (II) dichloride (15, 32 mg, 4.56 × 10−2 mmol) and triphenylphosphine (6, 32 mg, 1.2 × 10−4 mol) in 1,2-dimethoxyethane (DME, 10 mL) and aq. Na2CO3 (2.32 g Na2CO3 in 10 mL H2O) was saturated with argon. Thereafter, the mixture was heated at 82 °C for 12 h. The cooled mixture was added to water (70 mL) and extracted with CHCl3 (2 × 50 mL). The organic phase was separated, dried over anhydrous MgSO4 and evaporated in vacuo. The solid residue was taken up partially in CHCl3 (65 mL). The remaining solid was filtered off, washed with CHCl3 (3 × 20 mL) and air dried to yield the title compound (20). The filtrate was separated by column chromatography on silica gel (CH2Cl2) to give further dimethyl biphenyl-4,4′-diacrylate (20) as a “silvery” solid (altogether 580 mg, 90%); IR υ (KBr, cm−1): 2957, 1723, 1639, 1495, 1435, 1342, 1310, 1208, 1195, 1173, 984, 818, 739, 502, 493; δH (400 MHz, CDCl3): 3.82 (6H, s, 2 CO2CH3), 6.48 (2H, d, 3J = 16.0 Hz), 7.60 (4H, d, 3J = 8.0 Hz), 7.65 (4H, d, 3J = 8.0 Hz), 7.73 (2H, d, 3J = 16.0 Hz); δC (100.5 MHz, CDCl3): 51.8 (2 CH3, [2 CO2CH3]), 118.0 (2 CH), 127.4 (4 CH), 144.2 (2 CH), 133.9 (Cquat, 2C), 141.9 (Cquat, 2C), 167.4 (Cquat, 2 CO).
3. Results and Discussion
3-([1,1′-Biphenyl]-4-yl)acrylates find a number of uses. For instance, 3-(4′-alkoxy-[1,1′-biphenyl]-4-yl)acrylates have been presented as heat-resistant UV absorbers [] and have been used as intermediates for medicinally active compounds [,,]. For the most part, 3-biphenylacrylates have been prepared by Wittig or Horner–Emmons reactions of preformed, substituted biphenylcarbaldehydes [] or by Heck reactions of substituted halobiphenyls []. Thus, 3-(4′-alkoxy[1,1′-biphenyl]-4-yl)acrylates were prepared by consecutive but one-pot Suzuki cross-coupling–Heck reactions involving bromoiodobenzenes and acrylates []. Additionally, very often, the road to substituted methyl 3-([1,1′-biphenyl]-4-yl)acrylates involves a Suzuki cross-coupling reaction of bromocinnamates or bromocinnamic acid. For instance, methyl 3-(4′-methoxy[1,1′-biphenyl]-4-yl)acrylate is on the way to being used to make hydroxamic acid derivatives as anticancer drugs on the one hand [] and as histone dacetylase inhibitors [] on the other, prepared in this fashion. Additionally, methyl 3-(4′-methyl[1,1′-biphenyl]-4-yl)acrylate (24) was prepared by the reaction of p-tolylphenyboronic acid (23) with 4-bromocinnamic acid and with 4-chlorocinnamic acid, followed by subsequent esterification []. Ethyl 3-([1,1′-biphenyl]-4-yl)acrylate and ethyl 3-(3′-methyl[1,1′-biphenyl]-4-yl)acrylate were prepared by one-pot Suzuki–Horner–Emmons reaction in an ionic liquid protocol, where, however, the arylboronic acid could only be introduced after the Horner–Emmons reaction was complete []. We have published some prior work on the Suzuki cross-coupling–Wittig olefination to produce biphenylacrylates []. This contribution is seen as an extension of that work.
When mixtures of bromoarenes (5, 10), formylphenylboronic acids, e.g., 14, as central building blocks, and phosphorane 1a are heated in the presence of Pd(PPh3)2Cl2/PPh3 with Na2CO3 as base in a solvent mixture of DME/H2O, methyl 3-([1,1′-biphenyl]-4-yl)acrylates 2 are produced in acceptable yields (Scheme 2). Bromoarenes clearly give equally good results as iodoarenes []. In all cases, the E-olefin is formed as the main isomer (E/Z > 95/5), as evidenced by the coupling constant of the acrylic protons (3J = 16.0 Hz). Work-up is facile, with the cooled reaction mixture submitted to column chromatography on silica gel after aq. extraction. Care should be taken of the fact that some of the more symmetric 3-([1,1′-biphenyl]-4-yl)acrylates (2) are sparingly soluble in many solvents. In some instances, such as in the case of the preparation of 2e and 20, this can lead to a facilitation of the work-up as a simple filtration of the product directly from crude organic mixture is possible after aq. extraction of the reaction mixture. Due to the sparing solubility of some of the products in CH2Cl2, an eluant mixture of CH2Cl2/CHCl3 was used for the chromatographic separations.
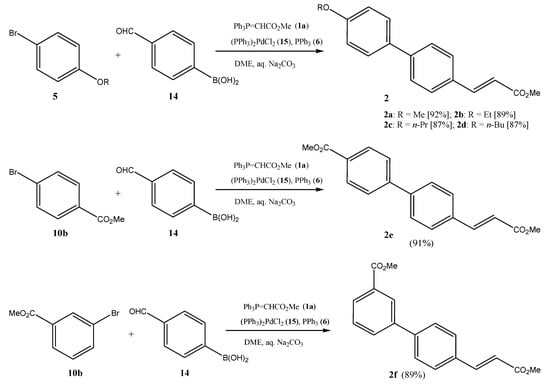
Scheme 2.
Suzuki cross-coupling/Wittig olefination one-pot transformation to methyl 3-(biphenyl)acrylates (2) using 4-bromophenylboronic acid (14) as the central building block.
It is possible to use both bromobenzaldehydes (11/18) and formylphenylboronic acid (14) as building blocks in the same reaction and perform a Suzuki cross-coupling/double Wittig olefination to prepare dimethyl biphenyl-diacrylates. Again, the E,E-isomers are formed, predominately (E/Z > 95/5) (Scheme 3).
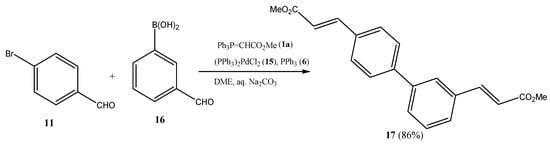
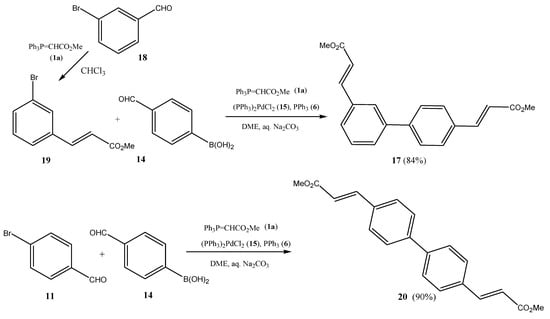
Scheme 3.
Suzuki cross-coupling/double Wittig olefination one-pot transformation to dimethyl biphenyl-diacrylates (17/20) using 4-bromobenzaldehyde (11) and formylphenylboronic acids (14/16) as central building blocks.
It is equally possible to use bromobenzaldhydes as central building blocks to prepare methyl 3-([1,1′-biphenyl]-3-yl)acrylates such as 22 and methyl 3-([1,1′-biphenyl]-4-yl)acrylates such as 24 (Scheme 4).
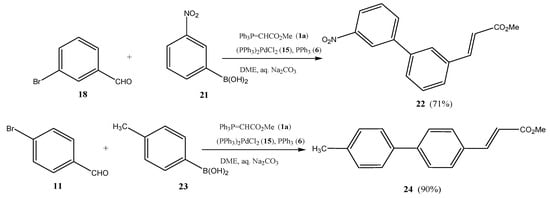
Scheme 4.
Suzuki cross-coupling/Wittig olefination one-pot transformation to methyl 3-(biphenyl)acrylates (2) using bromobenzaldehydes (11/18) as central building blocks.
The scope of the one-pot Suzuki cross-coupling–Wittig olefination reaction includes the use of halocinnamaldehydes such as 13 to give 5-(biphenyl)penta-2,4-dienoates such as compounds 26 and 27 (Scheme 5).
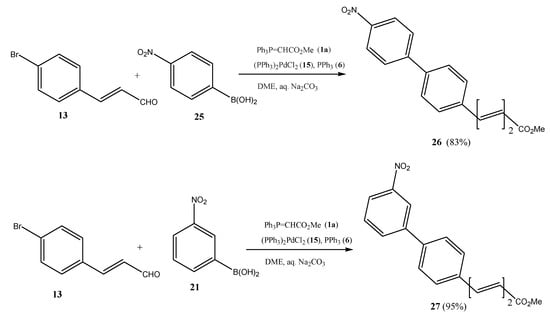
Scheme 5.
Preparation of methyl (E,E)-5-(nitrobiphenyl)penta-2,4-dienoates (26/27) by one Suzuki cross-coupling reaction/Wittig olefination in one pot.
Finally, the one-pot Suzuki–Wittig reaction can be augmented by a hydrolysis step. Previously, we have communicated a Wittig olefination hydrolysis one-pot transformation of benzaldehydes to cinnamic acids [], where the solvent system was not changed during the reaction but the temperature was elevated towards the end of the preparation. In the current case, the advertised solvent system—aq. Na2CO3/DME—was maintained during the one-pot Wittig–Suzuki reaction, where at the end of the preparation NaOH was added to the system and the reaction temperature increased (Scheme 6).
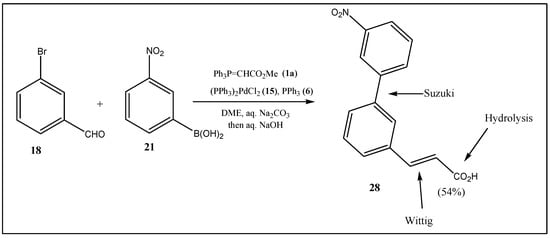
Scheme 6.
Transformations in Suzuki–Wittig hydrolysis in one pot lead to biphenylacrylic acids.
4. Conclusions
In conclusion, it can be said that alkyl 3-(biphenylacrylates) can be prepared facilely by one-pot Wittig olefination–Suzuki cross-coupling reaction, using either formylphenylboronic acids or bromobenzaldehydes, or both, as central building blocks. The use of bromoarenes leads to equally high yields as the use of iodoarenes. The reaction can be augmented by subsequent hydrolysis, also in one pot, to yield 3-(biphenyl)acylic acids. Similar one-pot reactions can be performed with bromocinnamaldehydes as the central building block to give 5-(biphenyl)penta-2,4-dienoates.
Author Contributions
Conceptualization, supervision, synthetic investigation, writing—original draft preparation, T.T.; synthetic investigation, A.H.; analytical work, V.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available on request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yamamoto, K.; Watanabe, W.; Ideta, K.; Mataka, S.; Thiemann, T. Combined Suzuki coupling—Wittig olefination reaction in aqueous medium. Z. Naturforschg. B 2005, 60B, 1299–1307. [Google Scholar] [CrossRef]
- Thiemann, T. Solventless Wittig reactions with fluorinated benzaldehydes. J. Chem. Res. 2007, 31, 336–341. [Google Scholar] [CrossRef]
- Thiemann, T.; Watanabe, M.; Tanaka, Y.; Mataka, S. One-Pot Wittig-olefination/Suzuki-reaction—The Compatibility of conjugated phosphoranes in Pd(0) catalysed C-C-bond forming reactions. New J. Chem. 2006, 30, 359–369. [Google Scholar] [CrossRef]
- Burmester, C.; Mataka, S.; Thiemann, T. Synthesis of non-symmetric divinylarenes by a Heck/Wittig reaction combination. Synth. Commun. 2010, 40, 3196–3208. [Google Scholar] [CrossRef]
- Watanabe, M.; Mataka, S.; Thiemann, T. One Pot Sonogashira-Coupling/Wittig olefination procedures. J. Chem. Res. 2005, 29, 636–639. [Google Scholar] [CrossRef]
- Satomura, M.; Takeda, A. (Fuji Shashin Film K. K., Japan). Preparation of Aromatic Inner Olefins as Heat-Resistant UV Absorbers. JP 04108763, 1992. Chem. Abstr. 1992, 117, 111238. [Google Scholar]
- Rao, J.M.R.; Arumugam, N.; Ansari, M.M.; Gudla, C.; Pachiyappan, S.; Ramalingam, M.; George, J.; Arul, G.F.; Bommegowda, Y.K.; Angupillai, S.K.; et al. Preparation of Methoxyiminophenylethyloxybenzyloxyphenylpropanoic Acid Derivatives and Analogs for Use as GPR40 Agonists (Connexios Life Sciences Pvt. Ltd., India). WO 2012011125, 2012. Chem. Abstr. 2012, 156, 202874. [Google Scholar]
- Pisano, C.; Giannini, G.; Vesci, L.; Zunino, F.; Dallavalle, S.; Merlini, L.; Penco, S. (Sigma-Tau Industrie Farmaceutiche Riunite S.p.A., Italy) Preparation of Biphenyl and Naphthylphenyl Hydroxamic Acids as Anticancer Drugs. PCT Int. Appl. WO 2007000383, 2007. Chem. Abstr. 2007, 146, 121694. [Google Scholar]
- Dallavalle, S.; Cincinelli, R.; Nannei, R.; Merlini, L.; Morini, G.; Penco, S.; Vesci, L.; Barbarino, M.; Zuco, V.; De Cesare, M.; Zunino, F. Design, synthesis, and evaluation of biphen-4-yl-acrylohydroxamic acid derivatives as histone deacetylase (HDAC) inhibitors. Eur. J. Med. Chem. 2009, 44, 1900–1912. [Google Scholar] [CrossRef] [PubMed]
- Collina, S.; Rossi, D.; Urbano, M.; Baraglia, A.C.; Azzolina, O. Microwave and polymer assisted synthesis of a small library of α,β-unsaturated methyl esters via the Wittig reaction. Lett. Org. Chem. 2007, 4, 384–387. [Google Scholar] [CrossRef]
- Jadhav, S.N.; Rode, C.V. An efficient palladium catalyzed Mizoroki-Heck cross-coupling in water. Green Chem. 2017, 24, 5958–5970. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, A.; Chen, W. Pd-catalyzed efficient one-pot sequential cross-coupling reactions of aryl dihalides. Org. Lett. 2008, 10, 3849–3852. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Ueda, M.; Miyaura, N. Palladium-catalyzed biaryl-coupling reaction of arylboronic acids in water using hydrophilic phosphine ligands. Tetrahedron 2002, 58, 5779–5787. [Google Scholar] [CrossRef]
- Savanur, H.M.; Kalkhambkar, R.G.; Laali, K. Piperidine-appended imidazolium ionic liquid as task-specific basic-IL for Suzuki and Heck reactions and for tandem Wittig-Suzuki, Wittig-Heck, Horner-Emmons-Suzuki, and Honer-Emmons-Heck protocols. Appl. Catal. A 2017, 543, 150–161. [Google Scholar] [CrossRef]
- Thiemann, T.; Elshorbagy, M.; Ahmadani, S.; Al Jasem, Y.; Al Azani, M.; al-Sulaibi, M.; al Hindawi, B. Facile, direct reaction of benzaldehydes to 3-phenyl-prop-2-enoic acids and 3-arylprop-2-ynoic acids in aqueous medium. Int. J. Org. Chem. 2016, 6, 126–141. [Google Scholar] [CrossRef][Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).