Structural Characterization by NMR Procedure of C4C1Pyrr TFSI Doped with Lithium TFSI Salt in Liquid and Gel States †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Gelation Procedure
3. Experimental Procedure
- Reverse detection probe 1H/13C/15N (standard tube 5 mm) with Z gradient.
- 1H/X multinuclear reverse detection probe (standard tube 5 mm) with Z gradient.
- X/1H multinuclear probe for 10 mm diameter tube.
- BACSTM 50-sample robot sample changer.
- Two waveform generators for selective pulses.
- Liquid N2 cooling device for low temperature experiments.
- Top Spin control software v. 1.3 under Linux Red-Hat Enterprise 5.1 Operating System.
4. Results
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kazemiabnavi, S.; Zhang, Z.; Thornton, K.; Banerjee, S. Electrochemical Stability Window of Imidazolium-Based Ionic Liquids as Electrolytes for Lithium Batteries. J. Phys. Chem. B 2016, 120, 5691–5702. [Google Scholar] [CrossRef] [PubMed]
- Salgado, J.; Parajó, J.J.; Villanueva, M.; Rodríguez, J.R.; Cabeza, O.; Varela, L.M. Liquid range of ionic liquid – Metal salt mixtures for electrochemical applications. J. Chem. Thermodyn. 2019, 134, 164–174. [Google Scholar] [CrossRef]
- Shimada, J.; Tsunashima, K.; Ue, M.; Iwasaki, K.; Tsuda, T.; Kuwabata, S.; Kanematsu, H.; Hirai, N.; Kogo, T.; Ogawa, A. Physical and Electrochemical Properties of Ionic Liquids Based on Quaternary Phosphonium Cations and Carboxylate Anions as Electrolytes. ECS Trans. 2017, 75, 105–111. [Google Scholar] [CrossRef]
- Hench, L.L.; West, J.K. The Sol-Gel Process. Chem. Rev. 1990, 90, 33–72. [Google Scholar] [CrossRef]
- Noor, S.A.M.; Bayley, P.M.; Forsyth, M.; MacFarlane, D.R. Ionogels based on ionic liquids as potential highly conductive solid state electrolytes. Electrochim. Acta 2013, 91, 219–226. [Google Scholar] [CrossRef]
- Parajó, J.J.; Villanueva, M.; Rodríguez, J.R.; Varela, L.M.; Salgado, J. Thermophysical characterization of ionogels of ethylamonium nitrate as advanced electrolytes for electrochemical devices. Proceeding of the 21st International Electronic Conference on Synthetic Organic Chemistry. ECSOC 2017; Available online: https://sciforum.net/paper/view/conference/4823 (accessed on 11 November 2020).
- Pavel, O.D.; Podolean, I.; Parvulescu, V.I.; Taylor, S.F.R.; Manyar, H.G.; Ralphs, K.; Goodrich, P.; Hardacre, C. Impact of SCILL catalysts for the S-S coupling of thiols to disulfides. Faraday Discuss. 2018, 206, 535–547. [Google Scholar] [CrossRef] [PubMed]
- Le Bideau, J.; Gaveau, P.; Bellayer, S.; Néouze, M.A.; Vioux, A. Effect of confinement on ionic liquids dynamics in monolithic silica ionogels: 1H NMR study. Phys. Chem. Chem. Phys. 2007, 9, 5419–5422. [Google Scholar] [CrossRef] [PubMed]

| Name | Molecular Weight (g mol−1) | Structure | Abbreviation CAS Number | Provenance Purity |
|---|---|---|---|---|
| 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)imide | 422.41 |  | C4C1Pyrr TFSI 223437-11-4 | Iolitec >0.99 |
| Lithium bis(trifluoromethylsulfonyl)imide | 287.09 | 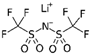 | Li TFSI 90076-65-6 | Acros Organics >0.99 |
| Tetraethoxysilane | 208.33 |  | TEOS 78-10-4 | Sigma Aldrich >0.98 |
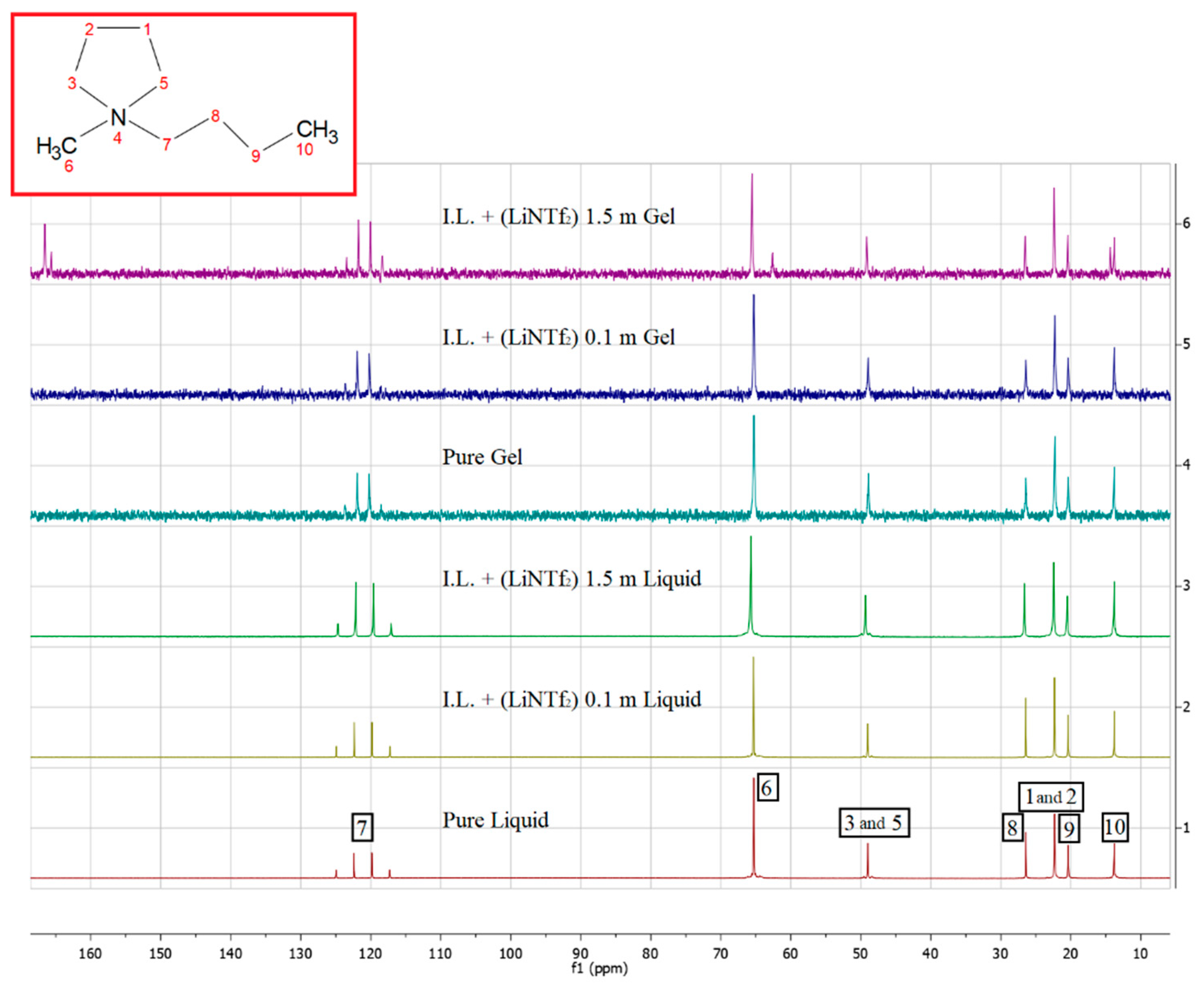
| 1H Spectra | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sample | 10 | 9 | 8 | 1, 2 | 6 | 7 | 3, 5 | |
| Pure IL (liquid) | 0.93 | 1.35 | 1.72 | 2.16 | 2.97 | 3.27 | 3.45 | |
| IL+Li TFSI 0.1m (liquid) | 0.93 | 1.34 | 1.72 | 2.16 | 2.97 | 3.27 | 3.45 | |
| IL+Li TFSI 1.5m (liquid) | 0.93 | 1.36 | 1.71 | 2.17 | 2.95 | 3.24 | 3.42 | |
| Pure IL (gel) | 0.93 | 1.35 | 1.72 | 2.16 | 2.97 | 3.28 | 3.46 | |
| IL+Li TFSI 0.1m (gel) | 0.93 | 1.35 | 1.72 | 2.16 | 2.97 | 3.28 | 3.45 | |
| IL+Li TFSI 1.5m (gel) | 0.93 | 1.36 | 1.73 | 2.18 | 2.98 | 3.27 | 3.45 | |
| 13C Spectra | ||||||||
| Sample | 10 | 9 | 1,2 | 8 | 3, 5 | 7 | 6 | CF3 |
| Pure IL (liquid) | 13.40 | 19.99 | 21.92 | 26.03 | 48.59 | 64.90 | 64.95 | 120.73 (Q) |
| IL+Li TFSI 0.1m (liquid) | 13.40 | 20.00 | 21.93 | 26.04 | 48.62 | 64.93 | 64.98 | 120.70 (Q) |
| IL+Li TFSI 1.5m (liquid) | 13.40 | 20.10 | 22.04 | 26.21 | 48.93 | 65.28 | 65.38 | 120.47 (Q) |
| Pure IL (gel) | 13.40 | 19.98 | 21.88 | 26.03 | 48.52 | 64.87 | UNDEF | 120.68 (Q) |
| IL+Li TFSI 0.1m (gel) | 13.40 | 19.97 | 21.89 | 26.02 | 48.55 | 64.90 | UNDEF | 120.68 (Q) |
| IL+Li TFSI 1.5m (gel) | 13.40 | 20.03 | 21.99 | 26.14 | 48.75 | 65.14 | UNDEF | 120.51 (Q) |
| 1H Spectra | ||||||||
|---|---|---|---|---|---|---|---|---|
| Sample | 10 | 9 | 8 | 1, 2 | 6 | 7 | 3, 5 | |
| Pure IL (liquid) | 19.29 | 25.26 | 28.19 | 20.49 | 12.49 | 25.37 | 22.59 | |
| IL+Li TFSI 0.1m (liquid) | 13.89 | 21.06 | 23.67 | 15.56 | 6.64 | 19.92 | 16.55 | |
| IL+Li TFSI 1.5m (liquid) | 24.66 | 32.62 | 35.26 | 24.86 | 15.81 | 31.94 | 32.72 | |
| Pure IL (gel) | 58.75 | 65.47 | 68.43 | 62.56 | 42.89 | 58.75 | 72.13 | |
| IL+Li TFSI 0.1m (gel) | 87.65 | 100.87 | 99.37 | 99.43 | 88.85 | 105.36 | 106.75 | |
| IL+Li TFSI 1.5m (gel) | 80.54 | 84.10 | 79.83 | 104.22 | 72.43 | 108.05 | 100.13 | |
| 13C Spectra | ||||||||
| Sample | 10 | 9 | 1,2 | 8 | 3,5 | 7 | 6 | CF3 |
| Pure IL (liquid) | 9.2 | 9.4 | 10.3 | 6.8 | 9.7 | 6.7 | 5.7 | 5.8; 5.4; 5.2; 5.2 |
| IL+Li TFSI 0.1m (liquid) | 5.5 | 5.7 | 6.7 | 4.3 | 9.1 | 5.3 | 6.2 | 3.9; 3.6; 3.5; 3.5 |
| IL+Li TFSI 1.5m (liquid) | 13.2 | 16.2 | 18.0 | 10.9 | 17.6 | 8.3 | 21.6 | 13.9; 9.4; 8.7; 11.6 |
| Pure IL (gel) | 23.7 | 30.8 | 31.2 | 33.4 | 28.8 | 40.3 | 21.3; 25.8; 25.6; 29.1 | |
| IL+Li TFSI 0.1m (gel) | 27.8 | 33.6 | 33.4 | 39.2 | 35.6 | 41.7 | 23.1; 25.3; 23.9; 25.5 | |
| IL+Li TFSI 1.5m (gel) | 21.9 | 24.1 | 23.1 | 24.3 | 27.0 | 30.8 | 24.8; 21.3; 23.6; 21.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallet, P.; Parajó, J.J.; Fernández-Míguez, L.; Sotuela, F.; Morcillo, Á.; Villanueva, M.; Cabeza, Ó.; Matveev, V.V.; Ievlev, A.V.; Tutukin, K.; et al. Structural Characterization by NMR Procedure of C4C1Pyrr TFSI Doped with Lithium TFSI Salt in Liquid and Gel States. Chem. Proc. 2021, 3, 115. https://doi.org/10.3390/ecsoc-24-08370
Vallet P, Parajó JJ, Fernández-Míguez L, Sotuela F, Morcillo Á, Villanueva M, Cabeza Ó, Matveev VV, Ievlev AV, Tutukin K, et al. Structural Characterization by NMR Procedure of C4C1Pyrr TFSI Doped with Lithium TFSI Salt in Liquid and Gel States. Chemistry Proceedings. 2021; 3(1):115. https://doi.org/10.3390/ecsoc-24-08370
Chicago/Turabian StyleVallet, Pablo, Juan José Parajó, Lois Fernández-Míguez, Félix Sotuela, Ángel Morcillo, María Villanueva, Óscar Cabeza, Vladimir V. Matveev, Alexandr V. Ievlev, Konstantin Tutukin, and et al. 2021. "Structural Characterization by NMR Procedure of C4C1Pyrr TFSI Doped with Lithium TFSI Salt in Liquid and Gel States" Chemistry Proceedings 3, no. 1: 115. https://doi.org/10.3390/ecsoc-24-08370
APA StyleVallet, P., Parajó, J. J., Fernández-Míguez, L., Sotuela, F., Morcillo, Á., Villanueva, M., Cabeza, Ó., Matveev, V. V., Ievlev, A. V., Tutukin, K., Varela, L. M., & Salgado, J. (2021). Structural Characterization by NMR Procedure of C4C1Pyrr TFSI Doped with Lithium TFSI Salt in Liquid and Gel States. Chemistry Proceedings, 3(1), 115. https://doi.org/10.3390/ecsoc-24-08370






