Two Symmetrical Squarylium Cyanine Dyes: Synthesis, Photophysics and Antifungal Activity in Saccharomyces cerevisiae †
Abstract
:1. Introduction
2. Results and Discussion
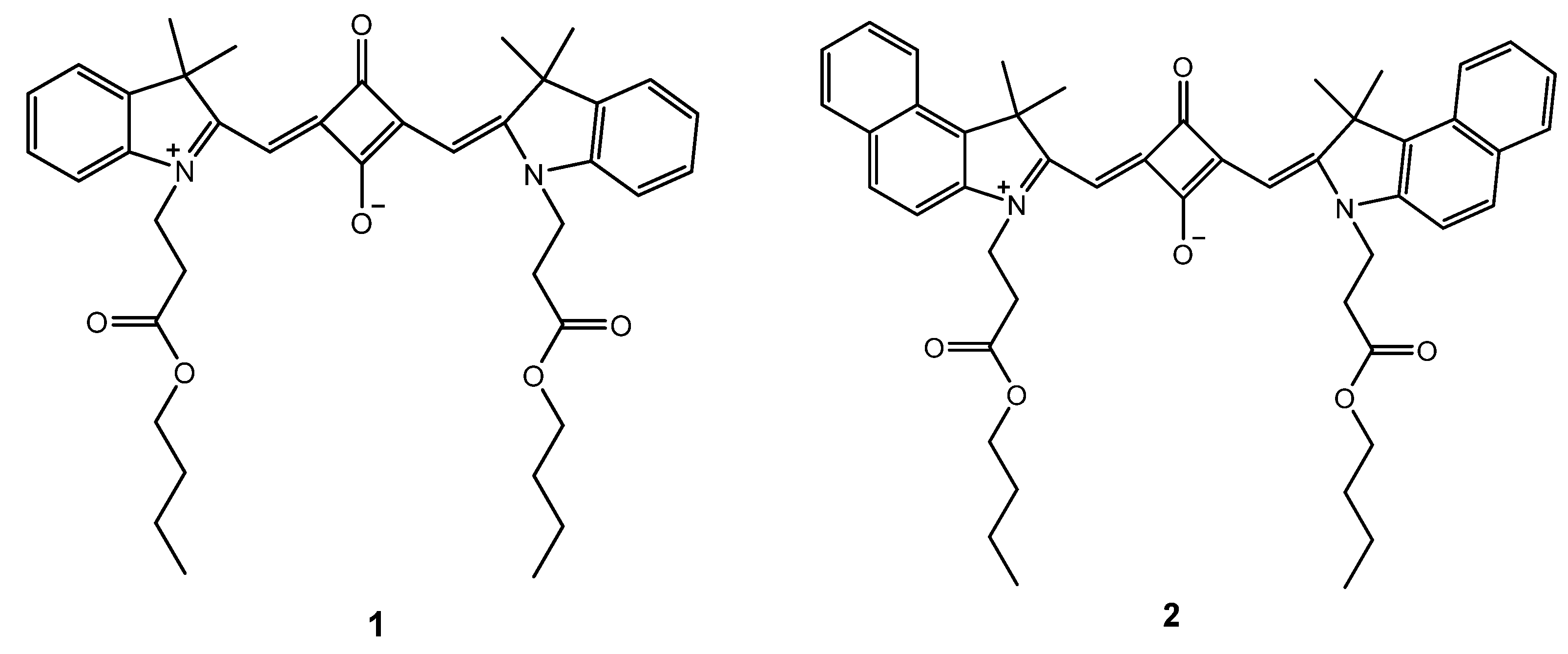
2.1. Synthesis of squarylium cyanine dyes 1 and 2
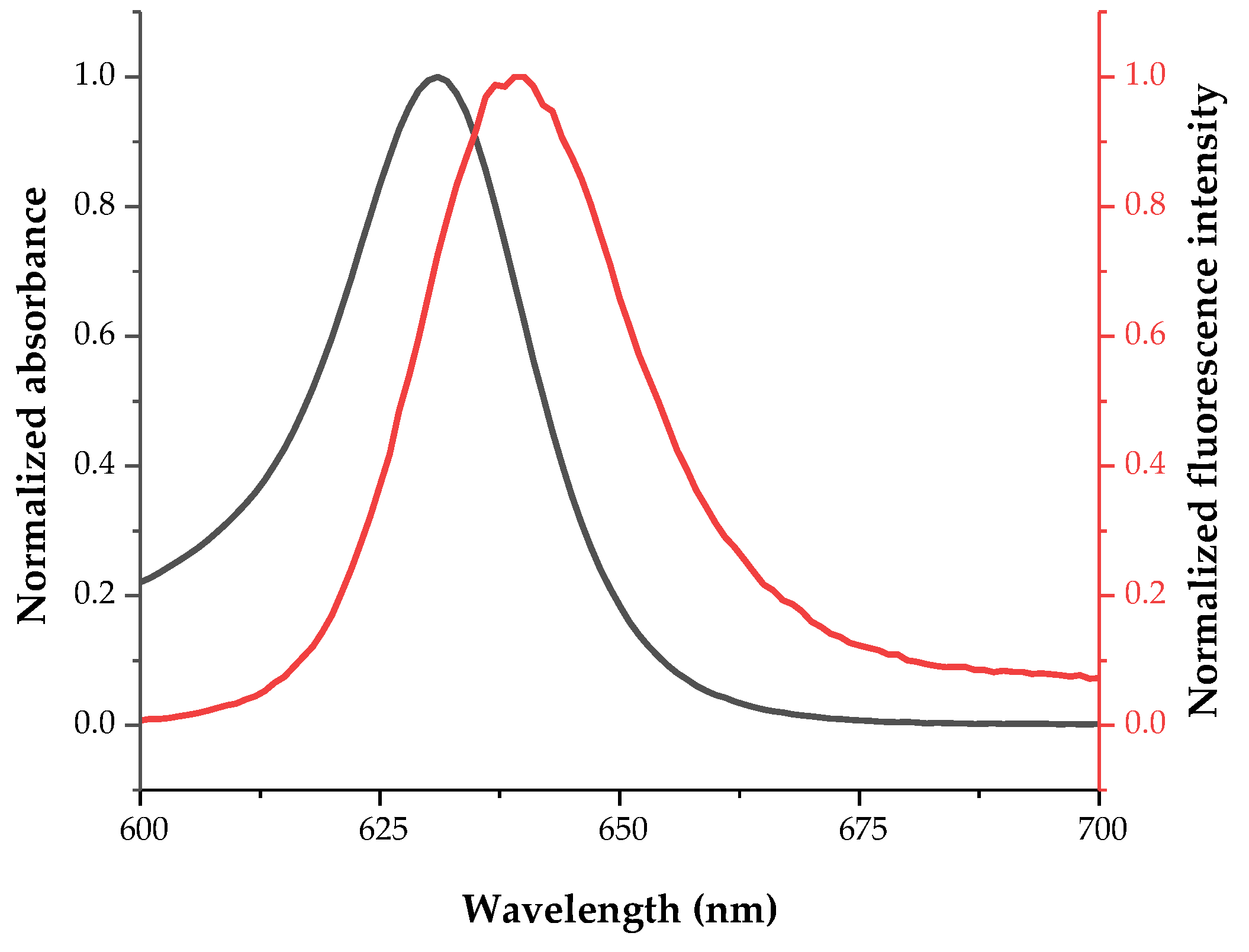
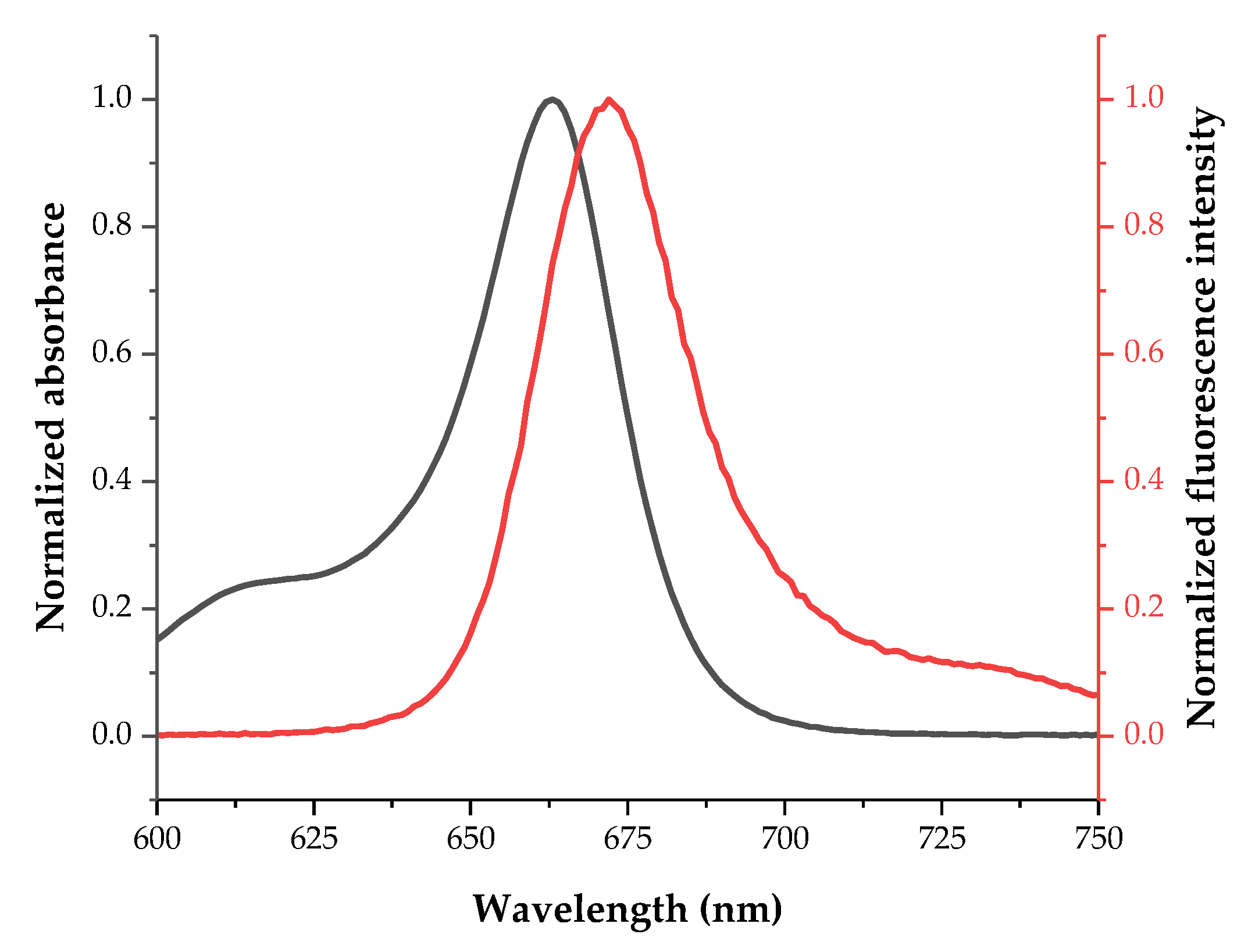
2.2. Fundamental Photophysics of Dyes 1 and 2
2.3. Antifungal Activity of Dyes 1 and 2
3. Experimental Section
3.1. Typical Procedure for the Synthesis of Dyes 1 and 2 (Illustrated for 1)
3.2. Antifungal Activity Assays
4. Conclusions
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reis:, L.V.; Serrano, J.P.; Almeida, P.; Santos, P.F. The synthesis and characterization of novel, aza-substituted squarylium cyanine dyes. Dyes Pigments 2009, 81, 197–202. [Google Scholar] [CrossRef]
- Yano, S.; Hirohara, S.; Obata, M.; Hagiya, Y.; Ogura, S.; Ikeda, A.; Kataoka, H.; Tanaka, M.; Joh, T. Current states and future views in photodynamic therapy. J. Photochem. Photobiol. C 2011, 12, 46–67. [Google Scholar] [CrossRef]
- Sreejith, S.; Carol, P.; Chithra, P.; Ajayaghosh, A. Squaraine dyes: A mine of molecular materials. J. Mater. Chem. 2008, 18, 264–274. [Google Scholar] [CrossRef]
- Pisoni, D.S.; Abreu, M.P.; Petzhold, C.L.; Rodembusch, F.S.; Campo, L.F. Synthesis, photophysical study and BSA association of water-insoluble squaraine dyes. J. Photochem. Photobiol. A 2013, 252, 77–83. [Google Scholar] [CrossRef]
- Martins, T.D.; Pacheco, M.L.; Boto, R.E.; Almeida, P.; Farinha, J.P.S.; Reis, L.V. Synthesis, characterization and protein-association of dicyanomethylene squaraine dyes. Dyes Pigments 2017, 147, 120–129. [Google Scholar] [CrossRef]
- Liu, L.; Chen, J.; Ku, Z.; Li, X.; Han, H. Unsymmetrical squaraine sensitizers containing auxiliary arylamine donor for NIR-harvesting on dye-sensitized solar cell. Dyes Pigments 2014, 106, 128–135. [Google Scholar] [CrossRef]
- Park, J.; Viscardi, G.; Barolo, C.; Barbero, N. Near-infrared Sensitization in Dye-sensitized Solar Cells. Chimia 2013, 67, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Avirah, R.R.; Jayaram, D.T.; Adarsh, N.; Ramaiah, D. Squaraine dyes in PDT: From basic design to in vivo demonstration. Org. Biomol. Chem. 2012, 10, 911–920. [Google Scholar] [CrossRef] [PubMed]
- Al-horaibi, S.A.; Asiri, A.M.; El-Shishtawy, R.M.; Gaikwad, S.T.; Rajbhoj, A.S. Synthesis and characterization of new squaraine dyes with bis-pendent carboxylic groups for dye-sensitized solar cells. J. Mol. Struct. 2019, 1195, 850–858. [Google Scholar] [CrossRef]
- Chen, G.; Sasabe, H.; Igarashi, T.; Hong, Z.; Kido, J. Squaraine dyes for organic photovoltaic cells. J. Mater. Chem. A 2015, 3, 14517–14534. [Google Scholar] [CrossRef]
- Fan, B.; Maniglio, Y.; Simeunovic, M.; Kuster, S.; Geiger, T.; Hany, R.; Nüesch, F. Squaraine Planar-Heterojunction Solar Cells. Int. J. Photoenergy 2009, 2009, 581068. [Google Scholar] [CrossRef]
- Gomes, V.S.D.; Gonçalves, H.M.R.; Boto, R.E.F.; Almeida, P.; Reis, L.V. Barbiturate squaraine dyes as fluorescent probes for serum albumins detection. J. Photochem. Photobiol. A 2020, 400, 112710. [Google Scholar] [CrossRef]
- Saikiran, M.; Sato, D.; Pandey, S.S.; Kato, T. Photophysical investigations of squaraine and cyanine dyes and their interaction with bovine serum albumin. J. Phys. Conf. Ser. 2016, 704, 012012. [Google Scholar] [CrossRef]
- He, Q.; Fan, X.; Sun, S.; Li, H.; Pei, Y.; Xu, Y. Highly selective turn-on detection of (strept)avidin based on self-assembled near-infrared fluorescent probes. RSC Adv. 2015, 5, 38571–38576. [Google Scholar] [CrossRef]
- Lima, E.; Ferreira, O.; Gomes, V.S.D.; Santos, A.O.; Boto, R.E.; Fernandes, J.R.; Almeida, P.; Silvestre, S.M.; Reis, L.V. Synthesis and in vitro evaluation of the antitumoral phototherapeutic potential of squaraine cyanine dyes derived from indolenine. Dyes Pigments 2019, 167, 98–108. [Google Scholar] [CrossRef]
- Lima, E.; Ferreira, O.; Silva, J.F.; Santos, A.O.; Boto, R.E.; Fernandes, J.R.; Almeida, P.; Silvestre, S.M.; Reis, L.V. Photodynamic activity of indolenine-based aminosquaraine cyanine dyes: Synthesis and in vitro photobiological evaluation. Dyes Pigments 2020, 174, 108024. [Google Scholar] [CrossRef]
- Friães, S.; Silva, A.M.; Boto, R.E.; Ferreira, D.; Fernandes, J.R.; Souto, E.B.; Almeida, P.; Ferreira, L.F.V.; Reis, L.V. Synthesis, spectroscopic characterization and biological evaluation of unsymmetrical aminosquarylium cyanine dyes. Bioorg. Med. Chem. 2017, 25, 3803–3814. [Google Scholar] [CrossRef] [PubMed]
- Ogunsipe, A.; Maree, D.; Nyokong, T. Solvent effects on the photochemical and fluorescence properties of zinc phthalocyanine derivatives. J. Mol. Struct. 2003, 650, 131–140. [Google Scholar] [CrossRef]
| Dye | Ethanol | ||||
|---|---|---|---|---|---|
| λabs (nm) | ε (M−1 cm−1) | λem (nm) | ΦF (%) | Δλ (nm) | |
| 1 | 631 | 385,684 | 639 | 81 | 8 |
| 2 | 663 | 324,591 | 672 | 26 | 9 |
| Dye | MIC (µM) | Log P |
|---|---|---|
| 1 | >100 | 1.70 |
| 2 | 50 | 4.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, V.S.D.S.D.; Ferreira, J.C.C.C.C.; Boto, R.E.F.E.F.; Almeida, P.; Sousa, M.J.M.F.J.M.F.; Reis, L.V.V.; Gonçalves, M.S.T.S.T. Two Symmetrical Squarylium Cyanine Dyes: Synthesis, Photophysics and Antifungal Activity in Saccharomyces cerevisiae . Chem. Proc. 2021, 3, 106. https://doi.org/10.3390/ecsoc-24-08423
Gomes VSDSD, Ferreira JCCCC, Boto REFEF, Almeida P, Sousa MJMFJMF, Reis LVV, Gonçalves MSTST. Two Symmetrical Squarylium Cyanine Dyes: Synthesis, Photophysics and Antifungal Activity in Saccharomyces cerevisiae . Chemistry Proceedings. 2021; 3(1):106. https://doi.org/10.3390/ecsoc-24-08423
Chicago/Turabian StyleGomes, Vanessa S. D. S. D., João C. C. C. C. Ferreira, Renato E. F. E. F. Boto, Paulo Almeida, Maria João M. F. João M. F. Sousa, Lucinda V. V. Reis, and M. Sameiro T. Sameiro T. Gonçalves. 2021. "Two Symmetrical Squarylium Cyanine Dyes: Synthesis, Photophysics and Antifungal Activity in Saccharomyces cerevisiae " Chemistry Proceedings 3, no. 1: 106. https://doi.org/10.3390/ecsoc-24-08423
APA StyleGomes, V. S. D. S. D., Ferreira, J. C. C. C. C., Boto, R. E. F. E. F., Almeida, P., Sousa, M. J. M. F. J. M. F., Reis, L. V. V., & Gonçalves, M. S. T. S. T. (2021). Two Symmetrical Squarylium Cyanine Dyes: Synthesis, Photophysics and Antifungal Activity in Saccharomyces cerevisiae . Chemistry Proceedings, 3(1), 106. https://doi.org/10.3390/ecsoc-24-08423










