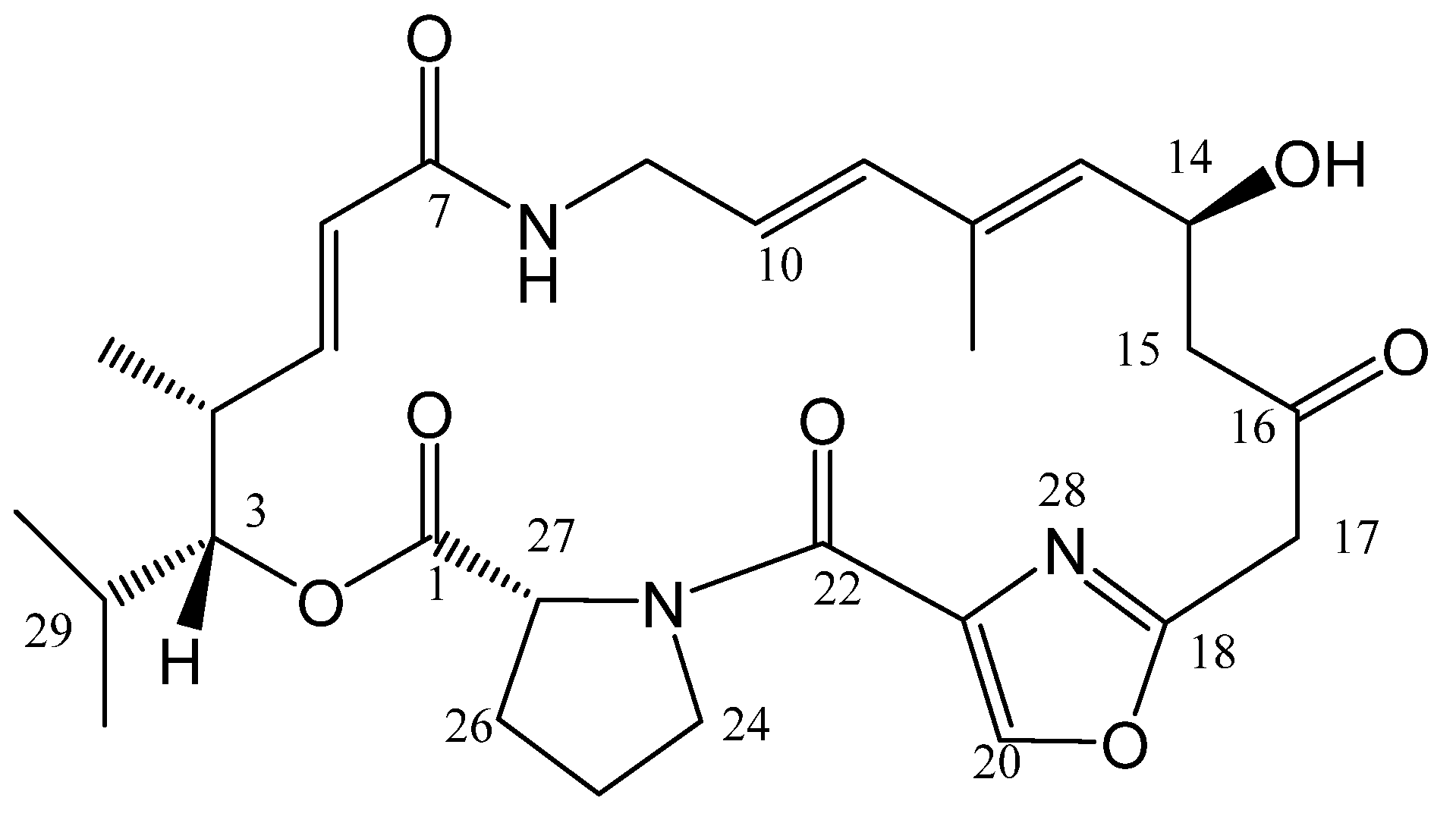
Tackling Pristinamycin IIB Problems: Synthetic Studies toward Some Fluorinated Analogs †
Abstract
Funding
References
- Mast, Y.; Wohlleben, W. Streptogramins-Two are better than one! Int. J. Med. Microbiol. 2014, 304, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Fass, R.J. In vitro activity of RP 59500, a semisynthetic injectable pristinamycin, against Staphylococci, Streptococci, and Enterococci. Antimicrob. Agents Chemother. 1991, 35, 553–559. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moellering, R.C. Discovering new antimicrobial agents. Int. J. Antimicrob. Agents 2011, 37, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Mezghani Maalej, S.; Malbruny, B.; Leclercq, R.; Hammami, A. Emergence of Staphylococcus aureus strains resistant to pristinamycin in Sfax (Tunisia). Pathol. Biol. 2012, 60, e71–e74. [Google Scholar] [CrossRef] [PubMed]
- Thal, L.A.; Zervos, M.J. Occurrence and epidemiology of resistance to virginiamycin and streptogramins. J. Antimicrob. Chemother. 1999, 43, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Cohen, R.J.; Fox, D.L.; Eubank, J.F.; Salvatore, R.N. Mild and efficient Cs2CO3-promoted synthesis of phosphonates. Tetrahedron Lett. 2003, 44, 8617–8621. [Google Scholar] [CrossRef]
- Marma, M.S.; Khawli, L.A.; Harutunian, V.; Kashemirov, B.A.; McKenna, C.E. Synthesis of α-fluorinated phosphonoacetate derivatives using electrophilic fluorine reagents: Perchloryl fluoride versus 1-chloromethyl-4-fluoro-1,4-diazoniabicyclo[2.2.2]octane bis(tetrafluoroborate) (Selectfluor®). J. Fluor. Chem. 2005, 126, 1467–1475. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chebieb, A.; Ziani-Cherif, C. Tackling Pristinamycin IIB Problems: Synthetic Studies toward Some Fluorinated Analogs. Chem. Proc. 2021, 3, 107. https://doi.org/10.3390/ecsoc-24-08388
Chebieb A, Ziani-Cherif C. Tackling Pristinamycin IIB Problems: Synthetic Studies toward Some Fluorinated Analogs. Chemistry Proceedings. 2021; 3(1):107. https://doi.org/10.3390/ecsoc-24-08388
Chicago/Turabian StyleChebieb, Assia, and Chewki Ziani-Cherif. 2021. "Tackling Pristinamycin IIB Problems: Synthetic Studies toward Some Fluorinated Analogs" Chemistry Proceedings 3, no. 1: 107. https://doi.org/10.3390/ecsoc-24-08388
APA StyleChebieb, A., & Ziani-Cherif, C. (2021). Tackling Pristinamycin IIB Problems: Synthetic Studies toward Some Fluorinated Analogs. Chemistry Proceedings, 3(1), 107. https://doi.org/10.3390/ecsoc-24-08388





