Photosynthesis-Inhibiting Activity of Methoxy-Substituted 3-Hydroxynaphthalene-2-Carboxanilides †
Abstract
:1. Introduction
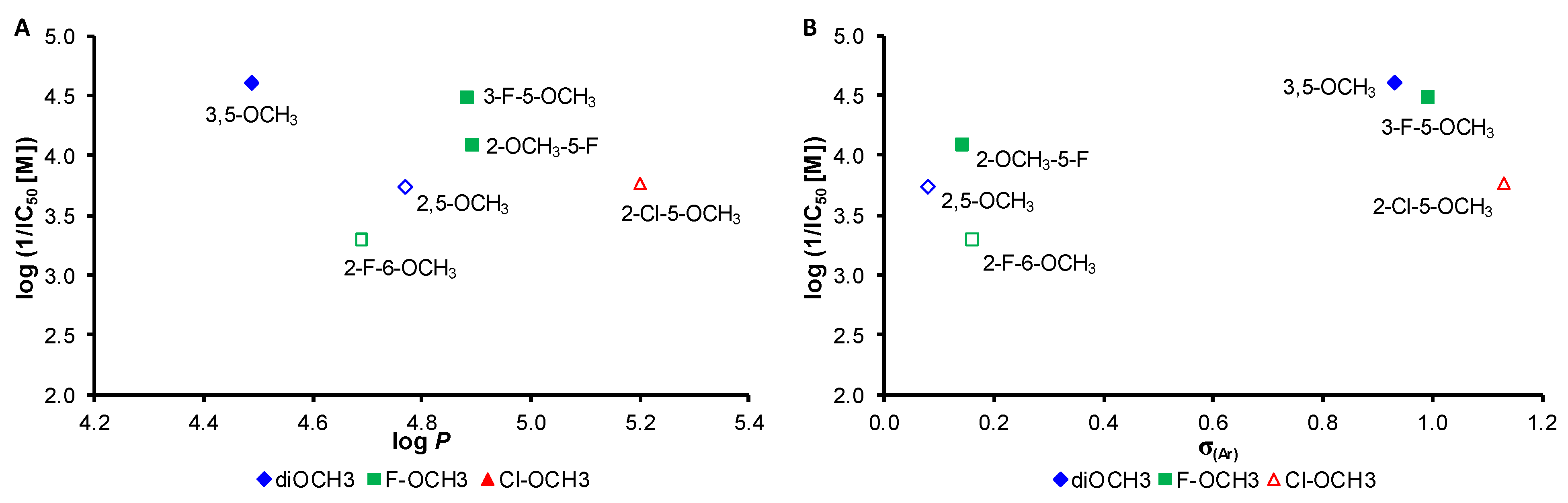
2. Results and Discussion
3. Experimental
3.1. General
3.2. Synthesis
3.3. Study of Photosynthetic Electron Transport (PET) Inhibition in Spinach Chloroplasts
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
References
- Duke, S. Overview of herbicide mechanisms of action. Environ. Health Perspect. 1990, 87, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Jablonkai, I. Molecular mechanism of action of herbicides. In Herbicides—Mechanisms and Mode of Action; Abd El-Ghany Hasaneen, M.N., Ed.; IntechOpen: Rijeka, Croatia, 2011; Chapter 1; Available online: https://www.intechopen.com/books/herbicides-physiology-of-action-and-safety/modes-of-action-of-different-classes-of-herbicides (accessed on 10 October 2020).
- Sherwani, S.I.; Arif, I.A.; Khan, H.A. Modes of action of different classes of herbicides. In Herbicides—Physiology of Action, and Safety; Price, A., Kelton, J., Sarunaite, L., Eds.; IntechOpen: Rijeka, Croatia, 2015; Chapter 8; Available online: https://www.intechopen.com/books/herbicides-physiology-of-action-and-safety/modes-of-action-of-different-classes-of-herbicides.
- Draber, W.; Tietjen, K.; Kluth, J.F.; Trebst, A. Herbicides in Photosynthesis Research. Angew. Chem. Int. Ed. 1991, 30, 1621–1633. [Google Scholar] [CrossRef]
- Tischer, W.; Strotmann, H. Relationship between inhibitor binding by chloroplasts and inhibition of photosynthetic electron transport. Biochim. Biophys. Acta (BBA)-Bioenerg. 1977, 460, 113–125. [Google Scholar] [CrossRef]
- Trebst, A.; Draber, W. Structure activity correlations of recent herbicides in photosynthetic reactions. In Synthesis of Pesticides Chemical Structure and Biological Activity Natural Products with Biological Activity; Elsevier BV: Amsterdam, The Netherlands, 1979; pp. 223–234. [Google Scholar]
- Bowyer, J.R.; Camilleri, P.; Vermaas, W.F.J. Herbicides, Topics in Photosynthesis; Baker, N.R., Percival, M.P., Eds.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 10, pp. 27–85. [Google Scholar]
- Izawa, S. Acceptors and donors for chloroplast electron transport. In Methods in Enzymology; Part, C., Colowick, P., Kaplan, N.O., Eds.; Academic Press: New York, NY, USA; London, UK, 1980; Volume 69, pp. 413–434. [Google Scholar]
- Lambreva, M.D.; Russo, D.; Polticelli, F.; Scognamiglio, V.; Antonacci, A.; Zobnina, V.; Campi, G.; Rea, G. Structure/function/dynamics of photosystem II plastoquinone binding sites. Curr. Protein Pept. Sci. 2014, 15, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, R.R.; Barros, M.V.D.A.; Bressan, G.C.; Siqueira, R.P.; Dos Santos, F.S.; Bertazzini, M.; Kiralj, R.; Ferreira, M.M.C.; Forlani, G. Synthesis, theoretical studies, and effect on the photosynthetic electron transport of trifluoromethyl arylamides. Pest Manag. Sci. 2017, 73, 2360–2371. [Google Scholar] [CrossRef]
- Lemke, T.L.; Williams, D.A. Foye’s Principles of Medicinal Chemistry, 7th ed.; Lippincott Williams & Wilkins and Wolters Kluwer: Baltimore, MD, USA, 2013. [Google Scholar]
- Laursen, J.S.; Engel-Andreasen, J.; Fristrup, P.; Harris, P.; Olsen, C.A. Cis–Trans amide bond rotamers in β-peptoids and peptoids: Evaluation of stereoelectronic effects in backbone and side chains. J. Am. Chem. Soc. 2013, 135, 2835–2844. [Google Scholar] [CrossRef]
- Good, N.E. Inhibitors of the Hill reaction. Plant Physiol. 1961, 36, 788–803. [Google Scholar] [CrossRef]
- Musiol, R.; Tabak, D.; Niedbala, H.; Podeszwa, B.; Jampilek, J.; Kralova, K.; Dohnal, J.; Finster, J.; Mencel, A.; Polanski, J. Investigating biological activity spectrum for novel quinoline analogues 2: Hydroxyquinolinecarboxamides with photosynthesis-inhibiting activity. Bioorg. Med. Chem. 2008, 16, 4490–4499. [Google Scholar] [CrossRef]
- Jampilek, J.; Kralova, K.; Pesko, M.; Kos, J. Ring-substituted 8-hydroxyquinoline-2-carboxanilides as photosystem II inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 3862–3865. [Google Scholar] [CrossRef]
- Opletalova, V.; Dolezel, J.; Kralova, K.; Pesko, M.; Kuneš, J.; Jampilek, J. Synthesis and characterization of (Z)-5-arylmethylidene-rhodanines with photosynthesis-inhibiting properties. Molecules 2011, 16, 5207–5227. [Google Scholar] [CrossRef]
- Fajkusova, D.; Pesko, M.; Keltosova, S.; Guo, J.; Oktabec, Z.; Marcela, V.; Kollar, P.; Coffey, A.; Csollei, J.; Kralova, K.; et al. Anti-infective and herbicidal activity of N-substituted 2-aminobenzothiazoles. Bioorg. Med. Chem. 2012, 20, 7059–7068. [Google Scholar] [CrossRef] [PubMed]
- Imramovsky, A.; Pesko, M.; Ferriz, J.M.; Kralova, K.; Vinsova, J.; Jampilek, J. Photosynthesis—inhibiting efficiency of 4-chloro-2-(chlorophenylcarbamoyl)phenyl alkylcarbamates. Bioorg. Med. Chem. Lett. 2011, 21, 4564–4567. [Google Scholar] [CrossRef] [PubMed]
- Kralova, K.; Perina, M.; Waisser, K.; Jampilek, J. Structure-activity relationships of N-benzylsalicylamides for inhibition of photosynthetic electron transport. Med. Chem. 2015, 11, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Pospisilova, S.; Kos, J.; Michnova, H.; Kapustikova, I.; Strharsky, T.; Oravec, M.; Moricz, A.M.; Bakonyi, J.; Kauerova, T.; Kollar, P.; et al. Synthesis and spectrum of biological activities of novel N-arylcinnamamides. Int. J. Mol. Sci. 2018, 19, 2318. [Google Scholar] [CrossRef]
- Kos, J.; Zadrazilova, I.; Pesko, M.; Keltosova, S.; Tengler, J.; Gonec, T.; Bobal, P.; Kauerova, T.; Oravec, M.; Kollar, P.; et al. Antibacterial and herbicidal activity of ring-substituted 3-hydroxynaphthalene-2-carboxanilides. Molecules 2013, 18, 7977–7997. [Google Scholar] [CrossRef]
- Kos, J.; Kapustikova, I.; Clements, C.; Gray, A.I.; Jampilek, J. 3-Hydroxynaphthalene-2-carboxanilides and their antitrypanosomal activity. Monatshefte für Chem.-Chem. Mon. 2018, 149, 887–892. [Google Scholar] [CrossRef]
- Kos, J.; Gonec, T.; Strharsky, T.; Oravec, M.; Jampilek, J. Preparation and photosynthesis-inhibiting activity of novel dihalogenated 3-hydroxynaphthalene-2-carboxanilides. In Multidisciplinary Digital Publishing Institute Proceedings; 2019; Volume 41, p. 30. Available online: https://www.mdpi.com/2504-3900/41/1/30/pdf (accessed on 10 October 2020).
- Bak, A.; Kos, J.; Michnova, H.; Gonec, T.; Pospisilova, S.; Kozik, V.; Cizek, A.; Smolinski, A.; Jampilek, J. Similarity-driven pharmacophore mapping for series of N-(disubstituted- phenyl)-3-hydroxynaphthalene-2-carboxamides. Int. J. Mol. Sci. 2020, 21, 6583. [Google Scholar] [CrossRef]
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, M.; Govender, R.; Keltosova, S.; Chambel, B.; Pereira, D.; Kollar, P.; Imramovsky, A.; et al. Antibacterial and herbicidal activity of ring-substituted 2-hydroxynaphthalene-1-carboxanilides. Molecules 2013, 18, 9397–9419. [Google Scholar] [CrossRef]
- Gonec, T.; Stranik, J.; Pesko, M.; Kos, J.; Oravec, M.; Kralova, K.; Jampilek, J. Photosynthesis-inhibiting activity of 1-[(2-chlorophenyl)carbamoyl]- and 1-[(2-nitrophenyl)carbamoyl]naphthalen-2-yl alkylcarbamates. Molecules 2017, 22, 1199. [Google Scholar] [CrossRef]
- Gonec, T.; Kos, J.; Zadrazilova, I.; Pesko, M.; Keltosova, S.; Tengler, J.; Bobal, P.; Kollar, P.; Cizek, A.; Kralova, K.; et al. Antimycobacterial and herbicidal activity of ring-substituted 1-hydroxynaphthalene-2-carboxanilides. Bioorg. Med. Chem. 2013, 21, 6531–6541. [Google Scholar] [CrossRef]
- Gonec, T.; Kos, J.; Pesko, M.; Dohanosova, J.; Oravec, M.; Liptaj, T.; Kralova, K.; Jampilek, J. Halogenated 1-hydroxynaphthalene-2-carboxanilides affecting photosynthetic electron transport in photosystem II. Molecules 2017, 22, 1709. [Google Scholar] [CrossRef] [PubMed]
- Michnova, H.; Pospisilova, S.; Gonec, T.; Kapustikova, I.; Kollar, P.; Kozik, V.; Musiol, R.; Jendrzejewska, I.; Vanco, J.; Travnicek, Z.; et al. Bioactivity of methoxylated and methylated 1-hydroxynaphthalene-2-carboxanilides: Comparative molecular surface analysis. Molecules 2019, 24, 2991. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Kos, J.; Nevin, E.; Govender, R.; Pesko, M.; Tengler, J.; Kushkevych, I.; Stastna, V.; Oravec, M.; Kollar, P.; et al. Preparation and biological properties of ring-substituted naphthalene-1-carboxanilides. Molecules 2014, 19, 10386–10409. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Zadrazilova, I.; Nevin, E.; Kauerova, T.; Pesko, M.; Kos, J.; Oravec, M.; Kollar, P.; Coffey, A.; O’Mahony, J.; et al. Synthesis and biological evaluation of N-alkoxyphenyl-3-hydroxynaphthalene-2-carboxanilides. Molecules 2015, 20, 9767–9787. [Google Scholar] [CrossRef] [PubMed]
- Gonec, T.; Kralova, K.; Pesko, M.; Jampilek, J. Antimycobacterial N-alkoxyphenyl-hydroxynaphthalenecarboxamides affecting photosystem II. Bioorg. Med. Chem. Lett. 2017, 27, 1881–1885. [Google Scholar] [CrossRef]
- Pesko, M.; Kos, J.; Kralova, K.; Jampilek, J. Inhibition of photosynthetic electron transport by 6-hydroxynaphthalene-2-carboxanilides. Indian J. Chem. B 2015, 54B, 1511–1517. [Google Scholar]
- Jampilek, J. Potential of agricultural fungicides for antifungal drug discovery. Expert Opin. Drug Discov. 2016, 11, 1–9. [Google Scholar] [CrossRef]
- Otevrel, J.; Mandelova, Z.; Pesko, M.; Guo, J.; Kralova, K.; Sersen, F.; Vejsova, M.; Kalinowski, D.S.; Kovacevic, Z.; Coffey, A.; et al. Investigating the spectrum of biological activity of ring-substituted salicylanilides and carbamoylphenylcarbamates. Molecules 2010, 15, 8122–8142. [Google Scholar] [CrossRef]
- Bak, A.; Pizova, H.; Kozik, V.; Svrckova, K.; Kos, J.; Treml, J.; Odehnalova, K.; Oravec, M.; Imramovsky, A.; Bobal, P.; et al. SAR-mediated similarity assessment of the property profile for new, silicon-based AChE/BChE inhibitors. Int. J. Mol. Sci. 2019, 20, 5385. [Google Scholar] [CrossRef]
- Kralova, K.; Masarovicova, E.; Jampilek, J. Plant responses to stress induced by toxic metals and their nanoforms. In Handbook of Plant and Crop Stress, 4th ed.; Pessarakli, M., Ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2019; pp. 479–522. [Google Scholar]

 | ||||
|---|---|---|---|---|
| Comp. | R | log P a | σ(Ar) a | PET Inhibition IC50 [µM] |
| 1 | 2,5-OCH3 | 4.77 | 0.08 | 183 |
| 2 | 3,5-OCH3 | 4.49 | 0.93 | 24.5 |
| 3 | 3-F-5-OCH3 | 4.88 | 0.99 | 31.6 |
| 4 | 2-F-6-OCH3 | 4.69 | 0.16 | 507 |
| 5 | 2-OCH3-5-F | 4.89 | 0.14 | 79.1 |
| 6 | 2-Cl-5-OCH3 | 5.20 | 1.13 | 171 |
| DCMU | – | – | – | 2.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kos, J.; Gonec, T.; Oravec, M.; Jampilek, J. Photosynthesis-Inhibiting Activity of Methoxy-Substituted 3-Hydroxynaphthalene-2-Carboxanilides. Chem. Proc. 2021, 3, 105. https://doi.org/10.3390/ecsoc-24-08295
Kos J, Gonec T, Oravec M, Jampilek J. Photosynthesis-Inhibiting Activity of Methoxy-Substituted 3-Hydroxynaphthalene-2-Carboxanilides. Chemistry Proceedings. 2021; 3(1):105. https://doi.org/10.3390/ecsoc-24-08295
Chicago/Turabian StyleKos, Jiri, Tomas Gonec, Michal Oravec, and Josef Jampilek. 2021. "Photosynthesis-Inhibiting Activity of Methoxy-Substituted 3-Hydroxynaphthalene-2-Carboxanilides" Chemistry Proceedings 3, no. 1: 105. https://doi.org/10.3390/ecsoc-24-08295
APA StyleKos, J., Gonec, T., Oravec, M., & Jampilek, J. (2021). Photosynthesis-Inhibiting Activity of Methoxy-Substituted 3-Hydroxynaphthalene-2-Carboxanilides. Chemistry Proceedings, 3(1), 105. https://doi.org/10.3390/ecsoc-24-08295







