Benzeneseleninic Acid in the Photo-Catalyzed Hydroxy-Selenylation of Styrenes †
Abstract
:1. Introduction
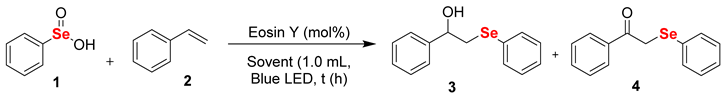
2. Results and Discussion
3. Conclusions
4. General Information
Author Contributions
Funding
Conflicts of Interest
References
- Tanini, D.; Degl’Innocenti, A.; Capperucci, A. Bis(trimethylsilyl)selenide in the Selective Synthesis of β-Hydroxy, β-Mercapto, and β-Amino Diorganyl Diselenides and Selenides Through Ring Opening of Strained Heterocycles. Eur. J. Org. Chem. 2014, 2015, 357–369. [Google Scholar] [CrossRef]
- Back, T.G.; Moussa, Z. Diselenides and Allyl Selenides as Glutathione Peroxidase Mimetics. Remarkable Activity of Cyclic Seleninates Produced in Situ by the Oxidation of Allyl ω-Hydroxyalkyl Selenides. J. Am. Chem. Soc. 2003, 125, 13455–13460. [Google Scholar] [CrossRef] [PubMed]
- Rigby, J.H.; Maharoof, U.S.M.; Mateo, M.E. Studies on the Narciclasine Alkaloids: Total Synthesis of (+)-Narciclasine and (+)-Pancratistatin. J. Am.Chem. Soc. 2000, 122, 6624–6628. [Google Scholar] [CrossRef]
- Azuma, H.; Tamagaki, S.; Ogino, K. Stereospecific Total Syntheses of Sphingosine and Its Analogues from l-Serine. J. Org. Chem. 2000, 65, 3538–3541. [Google Scholar] [CrossRef] [PubMed]
- Treadwell, E.M.; Neighbors, J.D.; Wiemer, D.F. A Cascade Cyclization Approach to Schweinfurthin B. Org. Lett. 2002, 4, 3639–3642. [Google Scholar] [CrossRef] [PubMed]
- Sharpless, K.B.; Lauer, R.F. Mild procedure for the conversion of epoxides to allylic alcohols. First organoselenium reagent. J. Am. Chem. Soc. 1973, 95, 2697–2699. [Google Scholar] [CrossRef]
- Rémion, J.; Dumont, W.; Krief, A. New regiospecific routes to olefins from β-hydroxy selenides. Tetrahedron Lett. 1976, 17, 1385–1388. [Google Scholar] [CrossRef]
- Sevrin, M.; Dumont, W.; Hevesi, L.; Krief, A. Transformation of selenides to alkylhalides new routes for homologization of primary alkylhalides. Tetrahedron Lett. 1976, 17, 2647–2650. [Google Scholar] [CrossRef]
- Gruttadauria, M.; Noto, R. Kinetic and thermodynamic control in the intramolecular hydroxyl capture of seleniranium ions. Tetrahedron Lett. 1999, 40, 8477–8481. [Google Scholar] [CrossRef]
- Yang, M.; Zhu, C.; Yuan, F.; Huang, Y.; Pan, Y. Enantioselective Ring-Opening Reaction of meso-Epoxides with ArSeH Catalyzed by Heterometallic Ti-Ga-Salen System. Org. Lett. 2005, 7, 1927–1930. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, S.; Ganesh, V. One-Pot Synthesis of β-Amino/β-Hydroxy Selenides and Sulfides from Aziridines and Epoxides. Synthesis 2009, 2009, 3267–3278. [Google Scholar] [CrossRef]
- Leng, T.; Wu, G.; Zhou, Y.-B.; Gao, W.-X.; Ding, J.; Huang, X.; Liu, M.; Wu, H.-Y. Silver-Catalyzed One-Pot Three-Component Selective Synthesis of β-Hydroxy Selenides. Adv. Synth. Catal. 2018, 360, 4336–4340. [Google Scholar] [CrossRef]
- Perin, G.; Santoni, P.; Barcellos, A.M.; Nobre, P.C.; Jacob, R.G.; Lenardao, E.J.; Santi, C. Selenomethoxylation of Alkenes Promoted by Oxone. Eur. J. Org. Chem. 2018, 2018, 1224–1229. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, S.; Yan, J. New Catalytic Method for the Synthesis of β-Hydroxy Selenides. Helv. Chim. Acta 2016, 99, 654–658. [Google Scholar] [CrossRef]
- Wang, X.-L.; Li, H.-J.; Yan, J. Iodine-mediated regioselective hydroxyselenylation of alkenes: Facile access to β-hydroxy selenides. Chin. Chem. Lett. 2018, 29, 479–481. [Google Scholar] [CrossRef]
- Hori, T.; Sharpless, K.B. Synthetic applications of arylselenenic and arylseleninic acids. Conversion of olefins to allylic alcohols and epoxides. J. Org. Chem. 1978, 43, 1689–1697. [Google Scholar] [CrossRef]
- Chenga, T.; Zheng, X.; Ke, Q. Ultrasound Assisted Ring-opening Reaction of Epoxides with 1,2-diphenyldiselenide. J. Chem. Res. 2011, 29, 522–524. [Google Scholar] [CrossRef]
- Vieira, A.A.; Azeredo, J.B.; Godoi, M.; Santi, C.; da Silva Júnior, E.N.; Braga, A.L. Catalytic Chalcogenylation under Greener Conditions: A Solvent-Free Sulfur- and Seleno-functionalization of Olefins via I2/DMSO Oxidant System. J. Org. Chem. 2015, 80, 2120–2127. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yuan, Y.; Yao, M.; Wang, H.; Wang, D.; Gao, M.; Yi-Hung Chen, Y.-H.; Lei, A. Electrochemical Aminoselenation and Oxyselenation of Styrenes with Hydrogen Evolution. Org. Lett. 2019, 21, 1297–1300. [Google Scholar] [CrossRef] [PubMed]
| Entry | Solvent | Time (h) | Yield 3 (%) b | Yield 4 (%) b |
|---|---|---|---|---|
| 1 | DMSO | 1 | 65 | Trace |
| 2 | DMSO | 2 | 73 | 11 |
| 3 | DMSO | 4 | 65 | 16 |
| 4 | DMSO | 24 | 56 | 21 |
| 5 | DCM | 2 | - | 18 |
| 6 | MeCN | 2 | 14 | 21 |
| 7 | THF | 2 | 15 | 4 |
| 8 | PEG 400 | 2 | 7 | Trace |
| 9 | DMF | 2 | 25 | 4 |
| 10 | Ethyl Acetate | 2 | 9 | - |
| 11 c | DMSO | 2 | 13 | Trace |
| 12 d | DMSO | 2 | 44 | 5 |
| 13 e | DMSO | 2 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penteado, F.; Bettanin, L.; Machado, K.; Lenardão, E.J. Benzeneseleninic Acid in the Photo-Catalyzed Hydroxy-Selenylation of Styrenes. Chem. Proc. 2020, 2, 9. https://doi.org/10.3390/ECCS2020-07758
Penteado F, Bettanin L, Machado K, Lenardão EJ. Benzeneseleninic Acid in the Photo-Catalyzed Hydroxy-Selenylation of Styrenes. Chemistry Proceedings. 2020; 2(1):9. https://doi.org/10.3390/ECCS2020-07758
Chicago/Turabian StylePenteado, Filipe, Luana Bettanin, Kethelyn Machado, and Eder J. Lenardão. 2020. "Benzeneseleninic Acid in the Photo-Catalyzed Hydroxy-Selenylation of Styrenes" Chemistry Proceedings 2, no. 1: 9. https://doi.org/10.3390/ECCS2020-07758
APA StylePenteado, F., Bettanin, L., Machado, K., & Lenardão, E. J. (2020). Benzeneseleninic Acid in the Photo-Catalyzed Hydroxy-Selenylation of Styrenes. Chemistry Proceedings, 2(1), 9. https://doi.org/10.3390/ECCS2020-07758






