Coke-Resistant Rh and Ni Catalysts Supported on γ-Al2O3 and CeO2 for Biogas Oxidative Steam Reforming †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Catalysts Preparation and Characterization
2.2. Experimental Apparatus and Procedures
3. Results and Discussion
3.1. Characterization
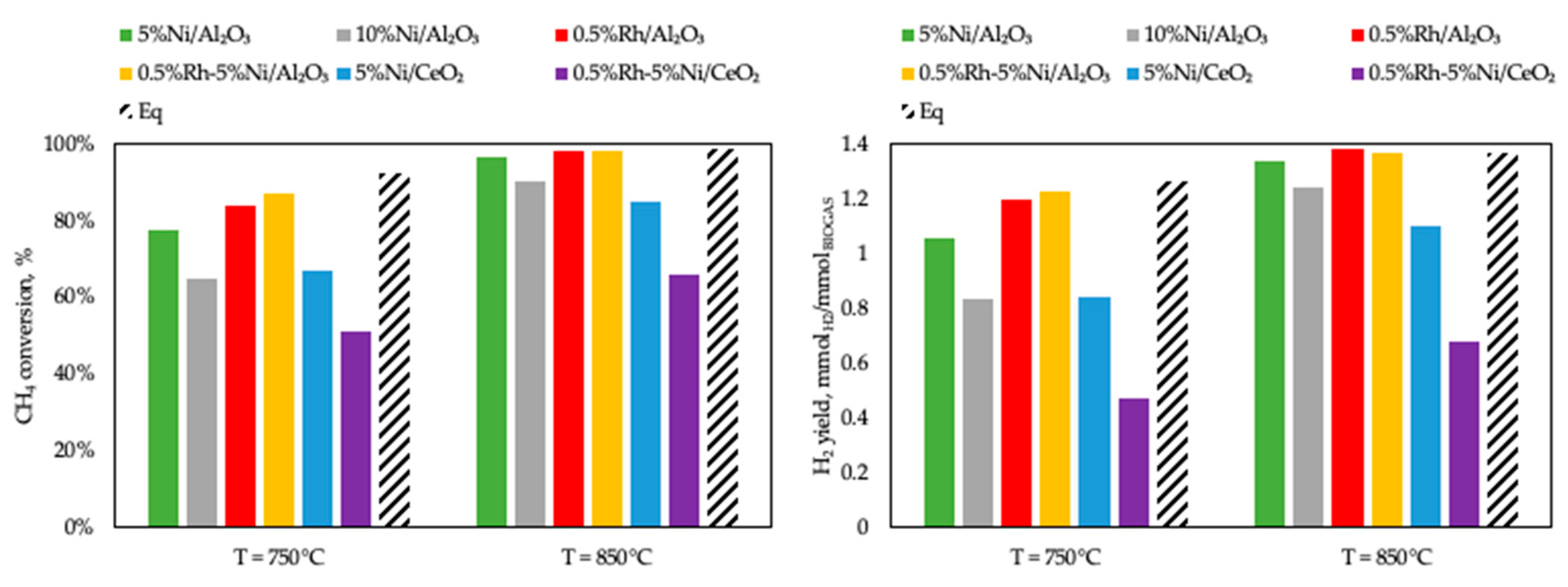
3.2. Activity Tests
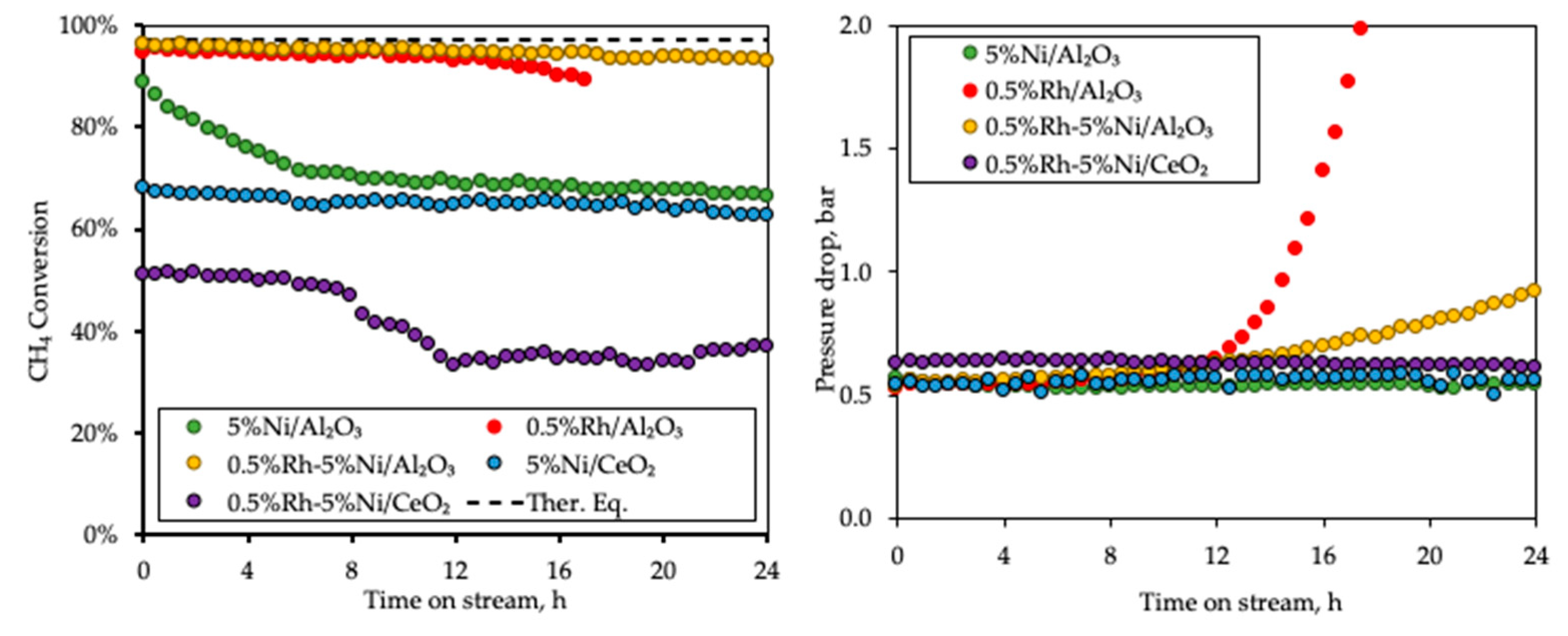
3.3. Deactivation Study
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kathiraser, Y.; Wang, Z.; Ang, M.L.; Mo, L.; Li, Z.; Oemar, U.; Kawi, S. Highly active and coke resistant Ni/SiO2 catalysts for oxidative reforming of model biogas: Effect of low ceria loading. J. CO2 Util 2017, 19, 284–295. [Google Scholar] [CrossRef]
- Tanios, C.; Bsaibes, S.; Gennequin, C.; Labaki, M.; Cazier, F.; Billet, S.; Tidahy, H.L.; Nsouli, B.; Aboukaïs, A.; Abi-Aad, E. Syngas production by the CO2 reforming of CH4 over Ni–Co–Mg–Al catalysts obtained from hydrotalcite precursors. Int. J. Hydrog. Energy 2017, 42, 12818–12828. [Google Scholar] [CrossRef]
- Song, C.; Pan, W. Tri-reforming of methane: A novel concept for catalytic production of industrially useful synthesis gas with desired H2/CO ratios. Catal. Today 2004, 98, 463–484. [Google Scholar] [CrossRef]
- Chein, R.Y.; Hsu, W.H. Analysis of Syngas Production from Biogas via the Tri-Reforming Process. Energies 2018, 11, 1075. [Google Scholar] [CrossRef]
- Izquierdo-Colorado, A.; Dębek, R.; Da Costa, P.; Gálvez, M.E. Excess-methane dry and oxidative reforming on Ni-containing hydrotalcite-derived catalysts for biogas upgrading into synthesis gas. Int. J. Hydrog. Energy 2018, 43, 11981–11989. [Google Scholar] [CrossRef]
- Zhao, X.; Ngo, H.T.; Walker, D.M.; Weber, D.; Maiti, D.; Cimenler, U.; Petrov, A.D.; Joseph, B.; Kuhn, J.N. Tri-reforming of surrogate biogas over Ni/Mg/ceria–zirconia/alumina pellet catalysts. Chem. Eng. Comm. 2018, 205, 1129–1142. [Google Scholar] [CrossRef]
- Luneau, M.; Gianotti, E.; Meunier, F.C.; Mirodatos, C.; Puzenat, E.; Schuurman, Y.; Guilhaume, N. Deactivation mechanism of Ni supported on Mg-Al spinel during autothermal reforming of model biogas. Appl. Catal. B Environ. 2017, 203, 289–299. [Google Scholar] [CrossRef]
- Roy, P.S.; Song, J.; Kim, K.; Kim, J.; Park, C.S.; Raju, A.S.K. Effects of CeZrO2–Al2O3 support composition of metal-foam-coated Pd–Rh catalysts for the steam-biogas reforming reaction. J. Ind. Eng. Chem. 2018, 62, 120–129. [Google Scholar] [CrossRef]
- Moral, A.; Reyero, I.; Alfaro, C.; Bimbela, F.; Gandía, L.M. Syngas production by means of biogas catalytic partial oxidation and dry reforming using Rh-based catalysts. Catal. Today 2018, 299, 280–288. [Google Scholar] [CrossRef]

| Label | Nominal Metal Loading wt% a | ED-XRF wt% b | SSA m2/g | YC % | CFR (mgcoke/gcat⋅gC,fed⋅h) | ||
|---|---|---|---|---|---|---|---|
| Ni | Rh | Ni | Rh | ||||
| 10%Ni/Al2O3 | 10 | - | 7.897 | - | 93 | ||
| 5%Ni/Al2O3 | 5 | - | 4.010 | - | 104 | 0.010 | 0.0046 |
| 0.5%Rh/Al2O3 | - | 0.5 | - | 0.423 | 96 | 0.230 | 0.2629 |
| 0.5%Rh-5%Ni/Al2O3 | 5 | 0.5 | 4.125 | 0.402 | 91 | 0.095 | 0.0793 |
| 5%Ni/CeO2 | 5 | - | 4.067 | - | 31 | ||
| 0.5%Rh-5%Ni/CeO2 | 5 | 0.5 | 4.102 | 0.399 | 15 | ||
| Al2O3 | 113 | ||||||
| CeO2 | 40 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Renda, S.; Ricca, A.; Palma, V. Coke-Resistant Rh and Ni Catalysts Supported on γ-Al2O3 and CeO2 for Biogas Oxidative Steam Reforming. Chem. Proc. 2020, 2, 10. https://doi.org/10.3390/ECCS2020-07588
Renda S, Ricca A, Palma V. Coke-Resistant Rh and Ni Catalysts Supported on γ-Al2O3 and CeO2 for Biogas Oxidative Steam Reforming. Chemistry Proceedings. 2020; 2(1):10. https://doi.org/10.3390/ECCS2020-07588
Chicago/Turabian StyleRenda, Simona, Antonio Ricca, and Vincenzo Palma. 2020. "Coke-Resistant Rh and Ni Catalysts Supported on γ-Al2O3 and CeO2 for Biogas Oxidative Steam Reforming" Chemistry Proceedings 2, no. 1: 10. https://doi.org/10.3390/ECCS2020-07588
APA StyleRenda, S., Ricca, A., & Palma, V. (2020). Coke-Resistant Rh and Ni Catalysts Supported on γ-Al2O3 and CeO2 for Biogas Oxidative Steam Reforming. Chemistry Proceedings, 2(1), 10. https://doi.org/10.3390/ECCS2020-07588







