Eco-Friendly Catalytic Aminoselenation of Alkenes: A Green Alternative for Obtaining Potentially Active Compounds †
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
References
- Ward, V.R. Aspects of Organoselenium Chemistry. Ph.D. Thesis, The University of Adelaide, Adelaide, Australia, 2012. [Google Scholar]
- Freudendahl, D.M. Green Chemistry with Selenium Reagents: Development of Efficient Catalytic Reactions. Angew. Chem. Int. Ed. 2009, 48, 8409–8411. [Google Scholar] [CrossRef] [PubMed]
- Freudendahl, D.M.; Shahzad, S.A.; Wirth, T. Recent Advances in Organoselenium Chemistry. Eur. J. Org. Chem. 2009, 2009, 1649–1664. [Google Scholar] [CrossRef]
- Shao, L. Recent progress in selenium-catalyzed organic reactions. Org. Chem. Front. 2019, 6, 2999–3041. [Google Scholar] [CrossRef]
- Li, H.; Liao, L.; Zhao, X. Organoselenium-Catalyzed Aza-Wacker Reactions: Efficient Access to Isoquinolinium Imides and an Isoquinoline N-Oxide. Synlett 2019, 30, 1688–1692. [Google Scholar] [CrossRef]
- Casas, J.S. Diorganotin (IV) complexes of pyridoxal thiosemicarbazone: Synthesis, spectroscopic properties and biological activity. J. Inorg. Biochem. 1998, 69, 283–292. [Google Scholar] [CrossRef]
- Teitz, Y. Inhibition of human immunodeficiency virus by N-methylisatin-beta 4′:4′-diethylthiosemicarbazone and N-allylisatin-beta-4′:4′-diallythiosemicarbazone. Antivir. Res. 1994, 24, 305–314. [Google Scholar] [CrossRef]
- Mugesh, G.; Du Mont, W.W.; Sies, H. Chemistry of biologically important synthetic organoselenium compounds. Chem. Rev. 2001, 101, 2125–2179. [Google Scholar] [CrossRef] [PubMed]
- Hili, R.; Yudin, A.K. Making carbon-nitrogen bonds in biological and chemical synthesis. Nat. Chem. Biol. 2006, 2, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Ricci, A. Amino Group Chemistry: From Synthesis to the Life Sciences, 1st ed.; Wiley-CVH: Weinheim, Germany, 2007. [Google Scholar]
- Zhang, H.; Gan, L.; Wang, H.; Zho, C. New Progress in Azole Compounds as Antimicrobial Agents. Mini-Rev. Med. Chem. 2017, 17, 122–166. [Google Scholar] [CrossRef] [PubMed]
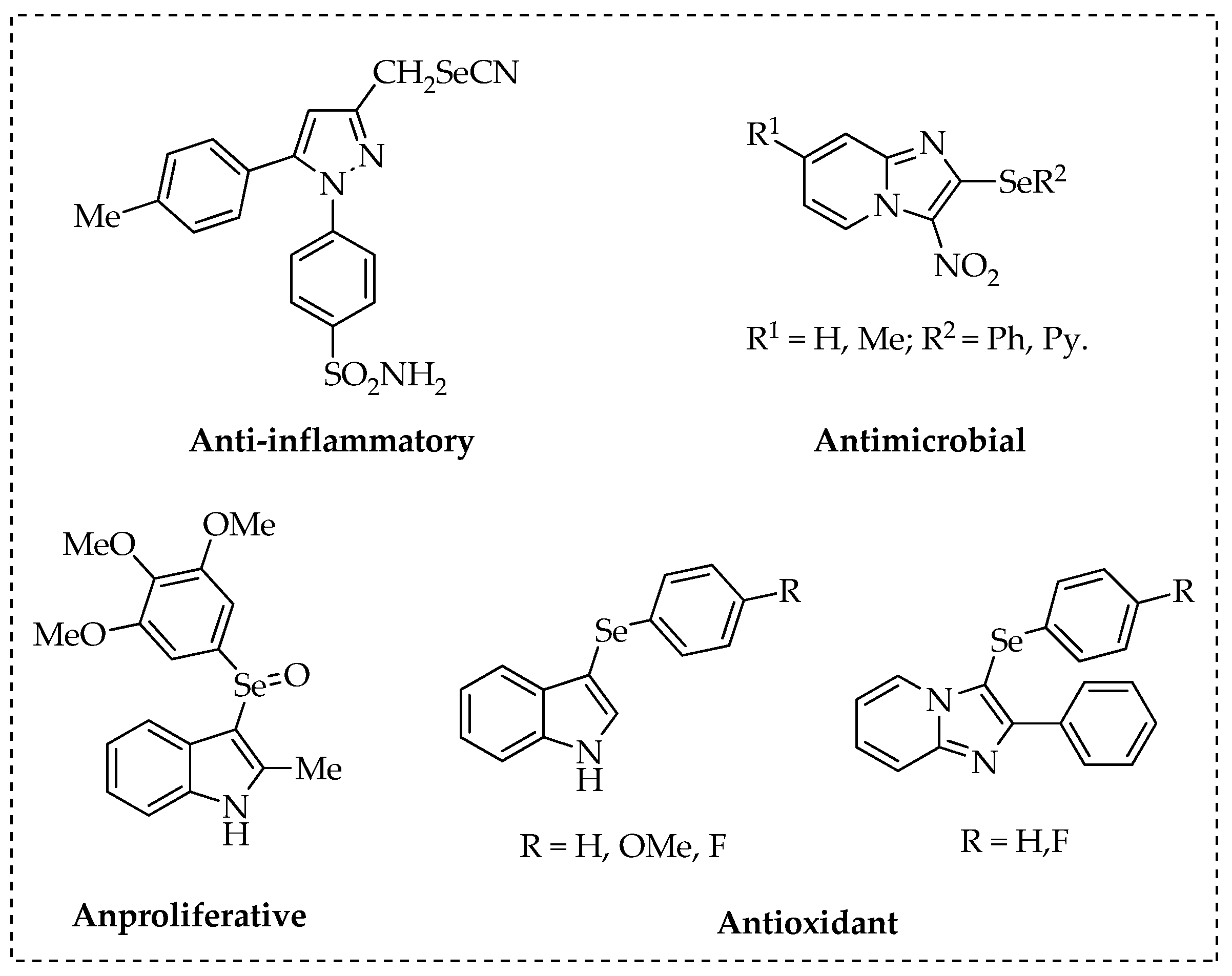
- Desai, D. Synthesis and evaluation of the anti-inflammatory properties of selenium-derivatives of celecoxib. Chem. Biol. Interact. 2010, 188, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S. Facile synthesis, structural evaluation, antimicrobial activity and synergistic effects of novel imidazo[1,2-a]pyridine based organoselenium compounds. Eur. J. Med. Chem. 2016, 123, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Wen, Z. 3-(3,4,5-Trimethoxyphenylselenyl)-1H-indoles and their selenoxides as combretastatin A-4 analogs: Microwave-assisted synthesis and biological evaluation. Eur. J. Med. Chem. 2015, 90, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Vieira, B.M. Ultrasound-Assisted Synthesis and Antioxidant Activity of 3-Selanyl-1 H-indole and 3-Selanylimidazo[1,2-a]pyridine Derivatives. J. Asian, Org. Chem. 2017, 6, 1635–1646. [Google Scholar] [CrossRef]

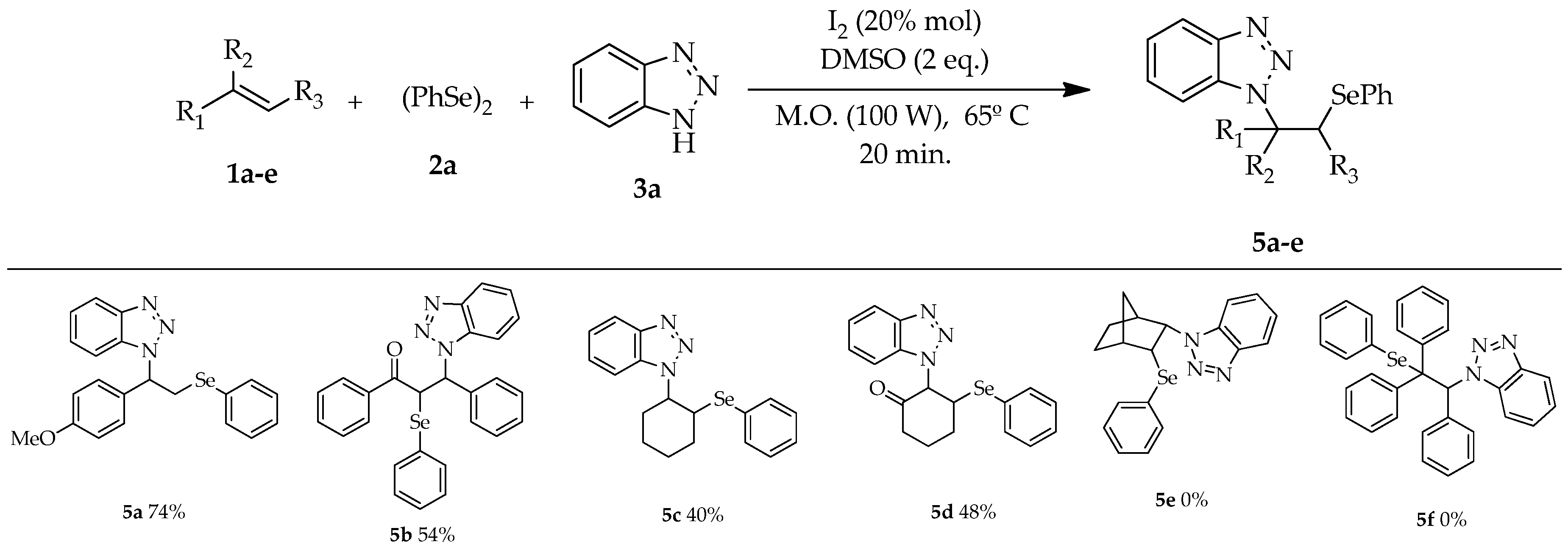
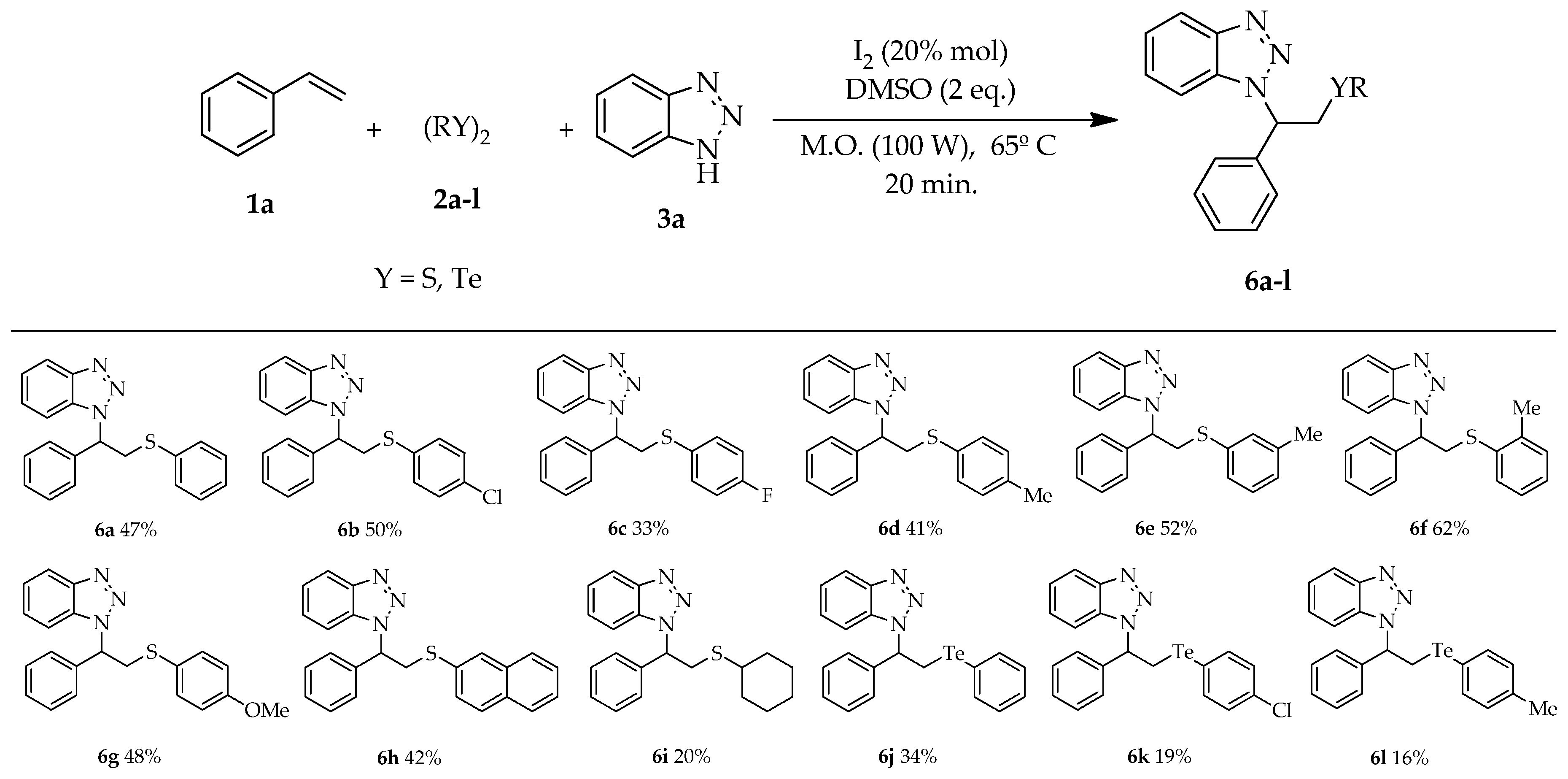

| # | DMSO (eq) | I2 (mol%) | T (°C) | Time (min) | Yield (b) (%) |
|---|---|---|---|---|---|
| 1 | 1 | 20 | 50 | 20 | 24 |
| 2 | 1 | 20 | 80 | 20 | 60 |
| 3 | 1 | 20 | 65 | 20 | 73 |
| 4 | 2 | 20 | 65 | 20 | 89 |
| 5 | 2 | 10 | 65 | 20 | 82 |
| 6 | 2 | 15 | 65 | 20 | 76 |
| 7 | 2 | 25 | 65 | 20 | 46 |
| 8 | 3 | 20 | 65 | 20 | 84 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, L.S.; Angelino, R.G.; Neto, J.S.S.; Leo, I.d.; Santi, C.; Nascimento, V. Eco-Friendly Catalytic Aminoselenation of Alkenes: A Green Alternative for Obtaining Potentially Active Compounds. Chem. Proc. 2020, 2, 8. https://doi.org/10.3390/ECCS2020-07580
Gomes LS, Angelino RG, Neto JSS, Leo Id, Santi C, Nascimento V. Eco-Friendly Catalytic Aminoselenation of Alkenes: A Green Alternative for Obtaining Potentially Active Compounds. Chemistry Proceedings. 2020; 2(1):8. https://doi.org/10.3390/ECCS2020-07580
Chicago/Turabian StyleGomes, Luana S., Rafaella G. Angelino, José S. S. Neto, Iris di Leo, Claudio Santi, and Vanessa Nascimento. 2020. "Eco-Friendly Catalytic Aminoselenation of Alkenes: A Green Alternative for Obtaining Potentially Active Compounds" Chemistry Proceedings 2, no. 1: 8. https://doi.org/10.3390/ECCS2020-07580
APA StyleGomes, L. S., Angelino, R. G., Neto, J. S. S., Leo, I. d., Santi, C., & Nascimento, V. (2020). Eco-Friendly Catalytic Aminoselenation of Alkenes: A Green Alternative for Obtaining Potentially Active Compounds. Chemistry Proceedings, 2(1), 8. https://doi.org/10.3390/ECCS2020-07580







