Paradoxical Behavior of Organodiselenides: Pro-Oxidant to Antioxidant †
Abstract
:1. Introduction
2. Bio-Chemical Activities under Cell-Free Conditions
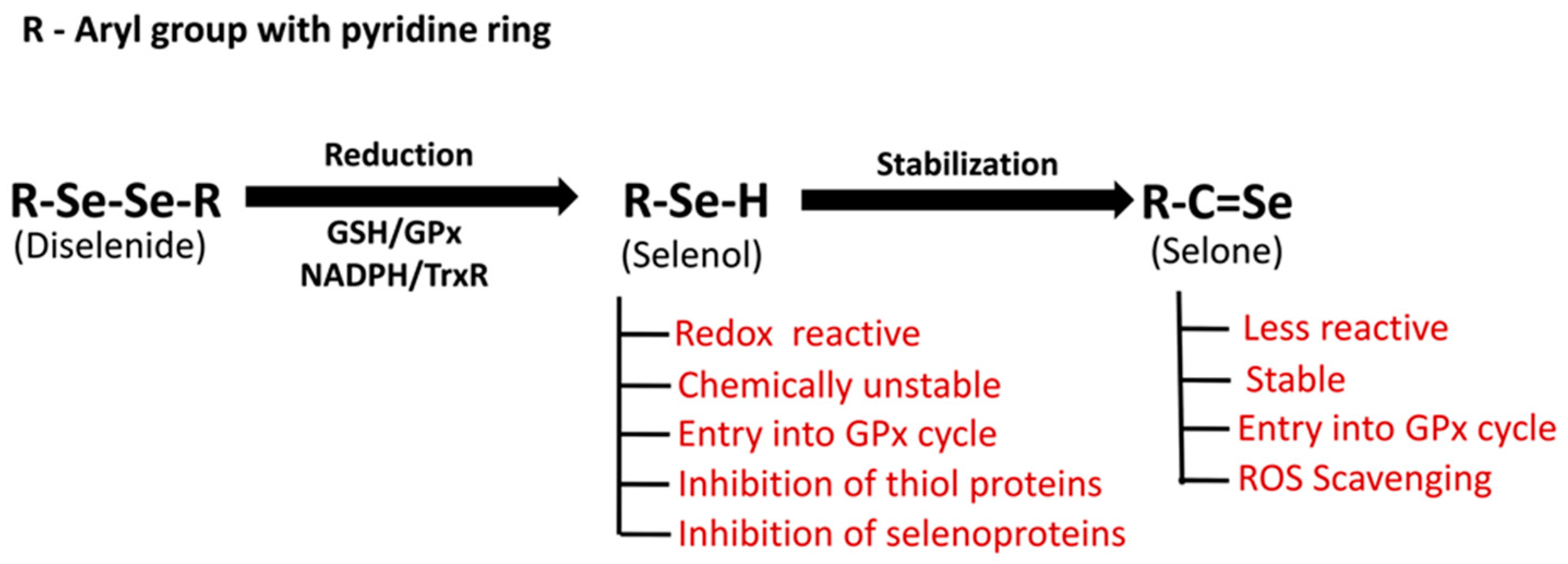
2.1. GPx-Like Activity
2.2. Substrate of TrxR
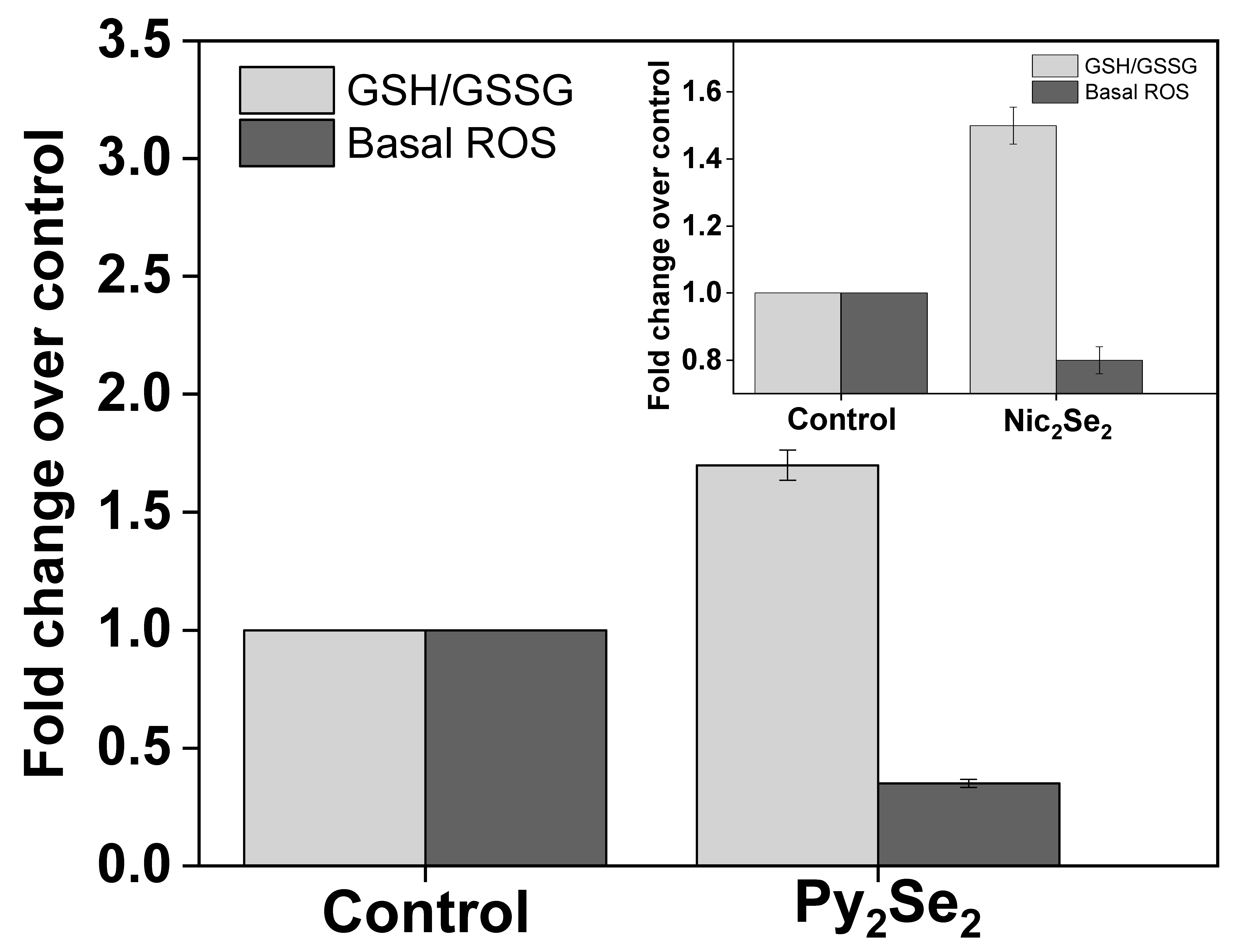
3. Comparative Cytotoxicity and Redox Modulation in Cells
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Papp, L.V.; Lu, J.; Holmgren, A.; Khanna, K.K. From selenium to selenoproteins: Synthesis, identity, and their role in human health. Antioxid. Redox. Signal. 2007, 9, 775–806. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.K.; Priyadarsini, K.I. (Eds.) Organoselenium Compounds in Biology and Medicine; RSC: London, UK, 2018. [Google Scholar]
- Alvarez-Perez, M.; Ali, W.; Marc, M.A.; Handzlik, J.; Dominguez-Alvarez, E. Selenides and Diselenides: A Review of Their Anticancer and Chemopreventive Activity. Molecules 2018, 23, 628. [Google Scholar] [CrossRef] [PubMed]
- Plano, D.; Baquedano, Y.; Ibáñez, E.; Jiménez, I.; Palop, J.A.; Spallholz, J.E.; Sanmartín, C. Antioxidant-prooxidant properties of a new organoselenium compound library. Molecules 2010, 15, 7292–7312. [Google Scholar] [CrossRef] [PubMed]
- Hodage, A.S.; Prabhu, C.P.; Phadnis, P.P.; Wadawale, A.; Priyadarsini, K.I.; Jain, V.K. Synthesis, characterization, structures and GPx mimicking activity of pyridyl and pyrimidyl based organoselenium compounds. J. Organomet. Chem. 2012, 720, 19–25. [Google Scholar] [CrossRef]
- Prabhu, C.P.; Phadnis, P.P.; Wadawale, A.; Priyadarsini, K.I.; Jain, V.K. Synthesis, characterization, structures and antioxidant activity of nicotinoyl based organoselenium compounds. J. Organomet. Chem. 2012, 713, 42–50. [Google Scholar] [CrossRef]
- Phadnis, P.P.; Kunwar, A.; Kumar, M.; Mishra, R.; Wadawale, A.; Priyadarsini, K.I.; Jain, V.K. Study of polymorphism in 2, 20-diselenobis(3-pyridinol). J. Organomet. Chem. 2017, 852, 1–7. [Google Scholar] [CrossRef]
- Chaudhari, K.R.; Kunwar, A.; Bhuvanesh, N.; Dey, S. Synthesis and anti-proliferative activities of amine capped Pd and Pt macrocycles of 4,4′-dipyridylselenides. New J. Chem. 2020, 44, 7329–7337. [Google Scholar] [CrossRef]
- Prabhu, C.P.; Singh, B.G.; Noguchi, M.; Phadnis, P.P.; Jain, V.K.; Iwaoka, M.; Priyadarsini, K.I. Stable selones in glutathioneperoxidase-like catalytic cycle of selenonicotinamide derivative. Org. Biomol. Chem. 2014, 12, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Raghuraman, M.; Verma, P.; Kunwar, A.; Phadnis, P.P.; Jain, V.K.; Priyadarsini, K.I. Cellular evaluation of diselenonicotinamide (DSNA) as a radioprotector against cell death and DNA damage. Metallomics 2017, 9, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, V.V.; Phadnis, P.P.; Kunwar, A. 2,2′-Dipyridyl diselenide (Py2Se2) induces G1 arrest and apoptosis in human lung carcinoma (A549) cells through ROS scavenging and reductive stress. Metallomics 2020, 12, 1253–1266. [Google Scholar] [CrossRef]
- Sudati, J.H.; Nogara, P.A.; Saraiva, R.A.; Wagner, C.; Alberto, E.E.; Braga, A.L.; Fachinetto, R.; Piquini, P.C.; Rocha, J.B.T. Diselenoamino acid derivatives as GPx mimics and as substrates of TrxR: In vitro and in silico studies. Org. Biomol. Chem. 2018, 16, 3777–3787. [Google Scholar] [CrossRef]
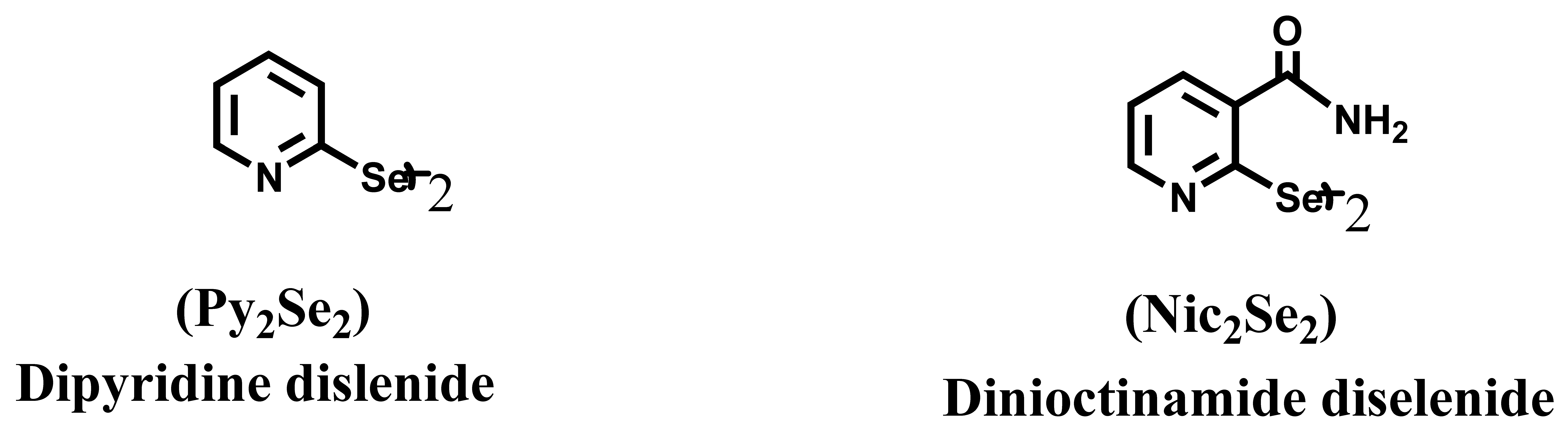

| Compounds | CHO | WI38 | A549 | MCF7 |
|---|---|---|---|---|
| Normal ovary epithelium | Normal lung fibroblast | Lung carcinoma | Breast carcinoma | |
| 2,2′-dipyridyl diselenide (Py2Se2) | ~6 µM | ~8 µM | ~5 µM | ~5 µM |
| 2,2′-diselenobis-[3-amidopyridine] (Nic2Se2) | >100 µM | ~70 µM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gandhi, V.V.; Priyadarsini, K.I.; Kunwar, A. Paradoxical Behavior of Organodiselenides: Pro-Oxidant to Antioxidant. Chem. Proc. 2020, 2, 6. https://doi.org/10.3390/ECCS2020-07552
Gandhi VV, Priyadarsini KI, Kunwar A. Paradoxical Behavior of Organodiselenides: Pro-Oxidant to Antioxidant. Chemistry Proceedings. 2020; 2(1):6. https://doi.org/10.3390/ECCS2020-07552
Chicago/Turabian StyleGandhi, Vishwa V., K. I. Priyadarsini, and A. Kunwar. 2020. "Paradoxical Behavior of Organodiselenides: Pro-Oxidant to Antioxidant" Chemistry Proceedings 2, no. 1: 6. https://doi.org/10.3390/ECCS2020-07552
APA StyleGandhi, V. V., Priyadarsini, K. I., & Kunwar, A. (2020). Paradoxical Behavior of Organodiselenides: Pro-Oxidant to Antioxidant. Chemistry Proceedings, 2(1), 6. https://doi.org/10.3390/ECCS2020-07552





