Abstract
Thermotoga maritima beta-fructosidases are enzymes that release beta-D-fructose from sucrose, raffinose, and fructan polymers such as inulin. The surfaces of beta-fructosidases 1UYP and 1W2T from Thermotoga maritima were studied in this work. It was showed that amino acids are not distributed equally on the surfaces of the enzymes. Several clusters of charged and hydrophobic residues were detected at pH 7.0. Such clusters were detected by calculation of the distances between them. It was determined that on surfaces of beta-fructosidases PDB ID: 1UYP and PDB ID: 1W2T, 96% and 95% of charged amino acids and also 50% and 42% of hydrophobic amino acids form clusters, respectively. Six clusters of charged amino acids on the surface of beta-fructosidase 1UYP and five clusters on the surface of beta-fructosidase 1W2T were detected. The composition of such clusters is presented. Both types of beta-fructosidase have three clusters of hydrophobic amino acids on their surface. These facts should be considered when choosing immobilization conditions. It was shown that a charged matrix is more promising for the immobilization of beta-fructosidases 1UYP and 1W2T from Thermotoga maritima due to the possibility of binding without any significant loss of activity due to their overlapping active center. Hydrophobic carriers are less promising due to the probable active site overlap. Such binding may have a loss of enzyme activity as a result.
1. Introduction
Thermotoga maritima beta-fructosidases are enzymes that release beta-D-fructose from sucrose, raffinose, and fructan polymers such as inulin. These biocatalysts are capable of hydrolyzing inulin, which can be obtained from cheap raw materials. Inulin (2,1-β-D fructan) is a linear homopolymer consisting of fructose units linked by β-2,1-bonds with a terminal glucose residue [1]. This polysaccharide is found in the roots and tubers of plants such as Jerusalem artichoke, chicory, leeks, onions, garlic, artichoke, wheat, banana, and dahlia [2].
Enzymes immobilization is a promising method of creating of industrial catalysts and medical agents. Due to their high specificity and activity, enzymes are very affective catalysts. They are less toxic than many different substances that can be used as catalytic agents. However, using soluble forms of enzymes has several limitations. For example, it is difficult to extract enzyme molecules from the complete product. In addition, soluble forms of enzymes can be degraded quickly in human organisms by its protective systems, so it is not possible to create stability drugs based on soluble forms of enzymes. Such limitations can be resolved by immobilization [3,4].
There are several methods of enzymes immobilization. More stability immobilized biocatalysts can be developed by covalent bonds. Nevertheless, this method of immobilization limits conformational mobility and decreases the enzymatic activity significantly. The development of a heterogeneous catalyst based on an enzyme immobilized by adsorption on a carrier is a promising method. In this case, weak interactions limit the conformational mobility to a lesser extent than covalent binding, which allows preserving the native structure of the enzyme and the highest activity as a result [5,6,7].
2. Methods
Beta-fructosidases from Thermotoga maritima PDB ID: 1UYP and PDB ID: 1W2T were chosen as the objects of this work. Molecular structures were visualized using the Maestro 10.3 software.
The degree of remoteness of amino acid residues from each other was a criterion for the grouping of amino acid residues into a local cluster. The residues were assigned to a certain cluster if the distance between the nearest atoms of these amino acids did not exceed 10 Å. The distance was calculated based on the formula:
3. Results and Discussion
Hydrophobic and charged residues are distributed not equally on the surfaces of molecules of beta-fructosidase presented in this work. There are several clusters of amino acids that were detected on the enzymes.
3.1. The Number and Composition of Amino Acids Clusters on Surfaces of the Enzymes
There are 83 charged amino acids presented on the surface of beta-fructosidase 1UYP, and 85 amino acids of such type presented on the surface of beta-fructosidase 1W2T. It was determined that 96% and 95% of charged amino acids form clusters, respectively. We detected 36 hydrophobic residues on the surface of the 1UYP enzyme and 33 residues of such type on the surface of 1W2T. It was shown than only 50% and 42% of hydrophobic residues form clusters on the surfaces of 1UYP and 1W2T, respectively. Six clusters of charged amino acids on the surface of beta-fructosidase 1UYP and five clusters on the surface of beta-fructosidase 1W2T were detected Both types of beta-fructosidase have three clusters of hydrophobic amino acids on their surface. The composition of amino acid clusters is shown in Table 1.

Table 1.
The composition of charged amino acids clusters.
Based on the above data, it can be assumed that the enzymes presented in this work may form stronger bonds with charged matrixes than with hydrophobic matrixes due to the closer co-location of charged residues than hydrophobic. However, clusters of charged amino acids contain residues with opposite charge, which may interfere with the binding of the enzyme to a matrix based on electrostatic interactions. These facts should be considered when choosing immobilization conditions.
3.2. Distribution of Amino Acids on the Surfaces of the Enzymes
As seen in Figure 1, there are clusters of amino acids in the region of the catalytic N-terminal domains of both types of beta-fructosidase. However, only such clusters were found for hydrophobic residues, which may indicate the probable binding of enzymes to the carrier in the region of the active center. Such binding may have a loss of enzyme activity as a result. Based on these data, it can be concluded that hydrophobic carriers are the least promising for the immobilization of fructosidase.
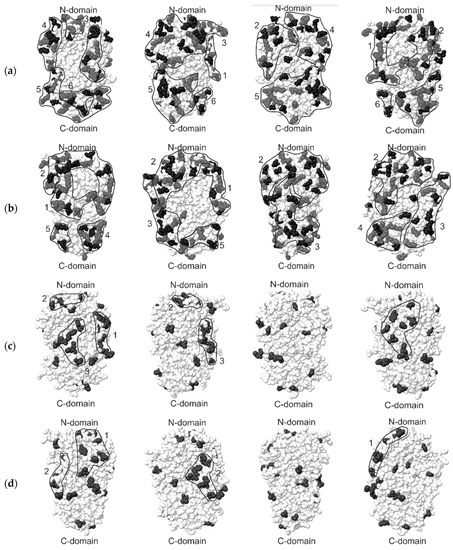
Figure 1.
Distribution of amino acids on the surfaces of beta-fructosidases 1UYP and 1W2T (molecules rotated 90 degrees): (a) The distribution of charged amino acid clusters on the surface of beta-fructosidase 1UY; (b) The distribution of charged amino acid clusters on the surface of beta-fructosidase 1W2; (c) The distribution of hydrophobic amino acid clusters on the surface of beta-fructosidase 1W2T; (d) The distribution of hydrophobic amino acid clusters on the surface of beta-fructosidase 1UYP.
There are clusters of charged amino acid that are localized at sites remote from the active site in the region of the C-terminal domain. For 1UYP, such clusters are No. 5 Glu286, Glu289, Asp296, Arg302, Lys303, Arg304, Lys305, Glu308, Lys311, Asp318, Lys320, Glu321, Glu333, Arg337, Arg351, Asp352, Glu353, Arg369, Lys370, Glu374, Asp375, Glu376, Arg380, Asp396, Lys416, Lys421, and No. 6 Glu341, Glu344, Arg360, Glu407. This may indicate the possibility of binding this enzyme to a charged carrier without a significant loss of activity.
For beta-fructosidase 1W2T, such clusters are No. 3 Arg196, Glu199, Lys221, Glu222, Glu228, Lys229, Glu286, Glu289, Glu333, Arg351, Asp352, Glu353, Lys370, Glu374, Asp375, Glu376, Arg380, Asp396, Lys416, Lys421, No. 4 Asp296, Arg302, Lys303, Arg304, Lys305, Glu308, Asp318, Lys320, Glu321, Glu426 and No. 5 Glu335, Arg337, Glu341, Arg360, and Glu407. However, cluster 3 is localized in the region of both N- and C-domains, which makes it possible to overlap the active center upon binding of this enzyme to a charged carrier. Nevertheless, it is still possible to bind this enzyme to a charged carrier without such an effect.
Based on these data, it can be concluded that the development of an immobilized biocatalyst based on these enzymes by its immobilization on charged carriers is promising.
4. Conclusions
Insoluble biocatalysts based on immobilized enzymes are promising catalytic agents for industry and medicine due to their high activity and stability. Immobilization based on weak interactions, such as hydrophobic and electrostatic interactions, makes it possible to save more activity than covalent bonds.
It was showed that charged carriers are more promising for the immobilization of beta-fructosidases 1UYP and 1W2T from Thermotoga maritima because of the presence of several clusters remote from the active site on its surfaces. The binding of the carrier’s matrix with such clusters may lead to immobilization of the enzymes without significant losses of activity.
Author Contributions
The authors have had equal contributions to the preparation and writing of the article. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Education and Science of the Russian Federation under state order No. FZGU-2020-0044.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Davies, G.; Henrissat, B. Structures and mechanisms of glycosyl hydrolases. Structure 1995, 3, 853–859. [Google Scholar] [CrossRef]
- Pons, T.; Naumoff, D.G.; Martínez-Fleites, C.; Hernández, L. Three acidic residues are at the active site of a β-propeller architecture in glycoside hydrolase families 32, 43, 62, and 68. Proteins 2004, 54, 424–432. [Google Scholar] [CrossRef] [PubMed]
- Cao, L. Immobilized enzymes: Science or art? Curr. Opin. Chem. Boil. 2005, 9, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilization in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.J.; Weissenborn, M.J.; Eyers, C.E.; Flitsch, S.L. Enzymatic reactions on immobilized substrates. Chem. Soc. Rev. 2013, 42, 6378–6405. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.; Ortiz, C.; Berenguer-Murcia, Á.; Torres, R.; Fernández-Lafuente, R. Modifying enzyme activity and selectivity by immobilization. Chem. Soc. Rev. 2013, 42, 6290–6307. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Palomo, J.M.; Fernandez-Lorente, G.; Guisan, J.M.; Fernandez-Lafuente, R. Improvement of enzyme activity, stability and selectivity via immobilization techniques. Enzym. Microb. Technol. 2007, 40, 1451–1463. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).