Abstract
Immobilized enzymes are the most sought-after preparations in the global market. They are used in medicine, veterinary medicine, the food industry, winemaking and brewing. The simplest method for immobilizing biocatalysts on insoluble carriers is the simple adsorption method. Its advantage is that it preserves the natural conformation of the enzyme, which slightly reduces its catalytic ability compared to the native form. In our study, we carried out the selection of optimal conditions for adsorption immobilization of acid-soluble chitosan (Mr = 350 kDa) enzymes of plant origin (ficin, papain and bromelain) on a matrix. Ficin (EC 3.4.22.3), papain (EC 3.4.22.2) and bromelain (EC 3.4.22.4) (Sigma) were chosen as the objects of study, azocasein (Sigma) was used as a substrate for hydrolysis and an acid-soluble high-molecular-weight chitosan (350 kDa) was used as an immobilization matrix, synthesized by Bioprogress CJSC. Suitable buffer systems for immobilization were identified by the optimal ratio of protein content and total and specific activity. Ficin is immobilized on a chitosan matrix using glycine buffer with a pH of 8.6. Glycine buffer with a pH of 8.6–10.5 is an optimal medium for sorption of papain on chitosan. Bromelain is immobilized on a chitosan matrix under Tris-glycine buffer with pH 8.5 conditions.
1. Introduction
Immobilized enzymes are the most sought-after preparations in the global market. They are used in medicine, veterinary medicine, the food industry, winemaking and brewing. The simplest method for immobilizing biocatalysts on insoluble carriers is the simple adsorption method. Its advantage is that it preserves the natural conformation of the enzyme, which slightly reduces its catalytic ability compared to the native form [1].
Cysteine proteolytic enzymes receive particular interest (ficin, bromelain and papain) due to their broad substrate specificity and the possibility of their use in all industries and production [2].
Chitosan is produced by deacetylation of chitin. It is a straight-chain polymer formed by β-(1,4)-linked glucosamine monomers; hydroxyl and amino groups are targets for chemical modifications aimed at obtaining suitable materials for various purposes [3,4].
The aim of this work was the selection of optimal conditions for adsorption immobilization of acid-soluble chitosan (Mr = 350 kDa) enzymes of plant origin (ficin, papain and bromelain) on a matrix.
2. Methods
Ficin (EC 3.4.22.3), papain (EC 3.4.22.2) and bromelain (EC 3.4.22.4) (Sigma-Aldrich, Darmstadt, Germany) were chosen as the objects of study, azocasein (Sigma) was used as a substrate for hydrolysis and an acid-soluble high-molecular-weight chitosan (350 kDa) was used as an immobilization matrix, synthesized by Bioprogress CJSC.
Immobilization of ficin, papain and bromelain on a chitosan matrix was carried out by the adsorption method. To 50 mg of chitosan, 1 mL of a buffer solution of the enzyme (ficin, papain or bromelain) was added and the mixture was incubated for 5 h with periodic stirring. At the end of the incubation, the formed precipitate was washed with 50 mM Tris-HCl buffer (pH 7.5) until there was no protein in the washings (a control was carried out on a spectrophotometer at λ = 280 nm).
The protein content in the immobilized enzymes was determined by the Lowry method [5].
3. Results and Discussion
During immobilization on the chitosan matrix, the largest amount of ficin was sorbed when using Tris-glycine buffer (pH 8.5), glycine buffer (pH 8.6–10.5) and borate buffer with the addition of KCl (pH 9.0); for bromelain and papain, it was when using borate buffer with the addition of KCl (pH 8.0–10.0), Tris-glycine buffer (pH 8.5–9.0) and glycine buffer (pH 8.6–10.5) (Figure 1).
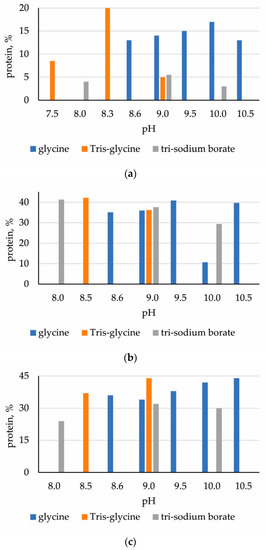
Figure 1.
Protein content in immobilized enzymes (in % of native biocatalyst): (a) ficin; (b) papain; (c) bromelain.
High total activity was demonstrated by preparations of immobilized ficin using glycine buffer with pH 8.6, Tris-glycine buffer with pH 7.5 or 8.5. When immobilized on chitosan, the total activity of papain was found to be higher when using a borate buffer supplemented with KCl at pH 8.0–10.0, glycine buffer at pH 8.6–10.5 and Tris-glycine buffer at pH 8.5–9.5. Bromelain sorbed on chitosan was the most active under immobilization conditions in Tris-glycine buffer with pH 8.5 (Figure 2).
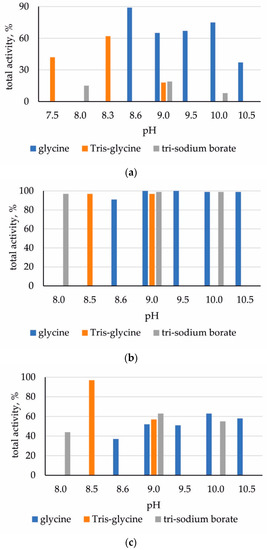
Figure 2.
Total activity of immobilized enzymes (in % of native biocatalyst): (a) ficin; (b) papain; (c) bromelain.
The highest specific activity during the immobilization of ficin on chitosan was revealed when using a glycine buffer with pH 8.6; during the sorption of papain—using glycine with pH 9.5–10.5, Tris-glycine 8.5–9.0 and borate with the addition of KCl with pH 9.0; and during adsorption of bromelain—when using Tris-glycine buffer with pH 8.5 (Figure 3).
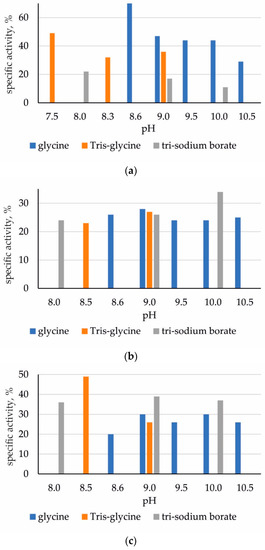
Figure 3.
Specific activity of immobilized enzymes (in % of native biocatalyst): (a) ficin; (b) papain; (c) bromelain.
4. Conclusions
The optimal buffer systems were selected for the adsorption immobilization of enzymes on the chitosan matrix; namely, glycine buffer pH 8.6 is promising for the sorption of ficin, glycine buffer pH 8.6–10.5 is promising for the adsorption of papain and Tris-glycine buffer pH 8.5—for the immobilization of bromelain.
5. Patents
Holyavka, M., Artyukhov, V., Koroleva, V. Method for obtaining heterogeneous preparation of various dispersities based on bromelain and chitosan. RU 2677232 C2. Date of publication: 16.01.2019 Bull. № 2.
Author Contributions
All authors made equal contributions in the preparation and writing of the article.
Funding
This work was financially supported in the form of a grant from the President of the Russian Federation for state support to young Russian scientists—Doctors of Sciences (MD-1982.2020.4. Agreement 075-15-2020-325).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Feijoo-Siota, L.; Villa, T.G. Native and biotechnologically engineered plant proteases with industrial applications. Food Bioprocess Technol. 2011, 4, 1066–1088. [Google Scholar] [CrossRef]
- Tavano, O.L.; Berenguer-Murcia, A.; Secundo, F.; Fernandez-Lafuente, R. Biotechnological applications of proteases in food technology. Compr. Rev. Food Sci. Food Saf. 2018, 17, 412–436. [Google Scholar] [CrossRef] [PubMed]
- Fini, A.; Orienti, I. The Role of Chitosan in Drug Delivery. Current and Potential Applications. Am. J. Drug Deliv. 2003, 1, 43–59. [Google Scholar] [CrossRef]
- Jafarizadeh-Malmiri, H.; Ghaz-Jahanian, M.A.; Berenjian, A. Potential applications of chitosan nanoparticles as novel supports in enzyme immobilization. Am. J. Biochem. Biotechnol. 2012, 8, 203–219. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Faar, A.L.; Randall, R.J. Protein measurement with folin-phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).