Fenton Degradation of Ofloxacin Using a Montmorillonite-Fe3O4 Composite †
Abstract
:1. Introduction
2. Experimental
2.1. Synthesis of Fe3O4-Montmorillonite (FeM) Composites
2.2. Fenton Reaction
3. Results and Discussion
3.1. Characterization of Bare Montmorillonite and Fe3O4-Montmorillonite Composites
3.2. Fenton Reaction
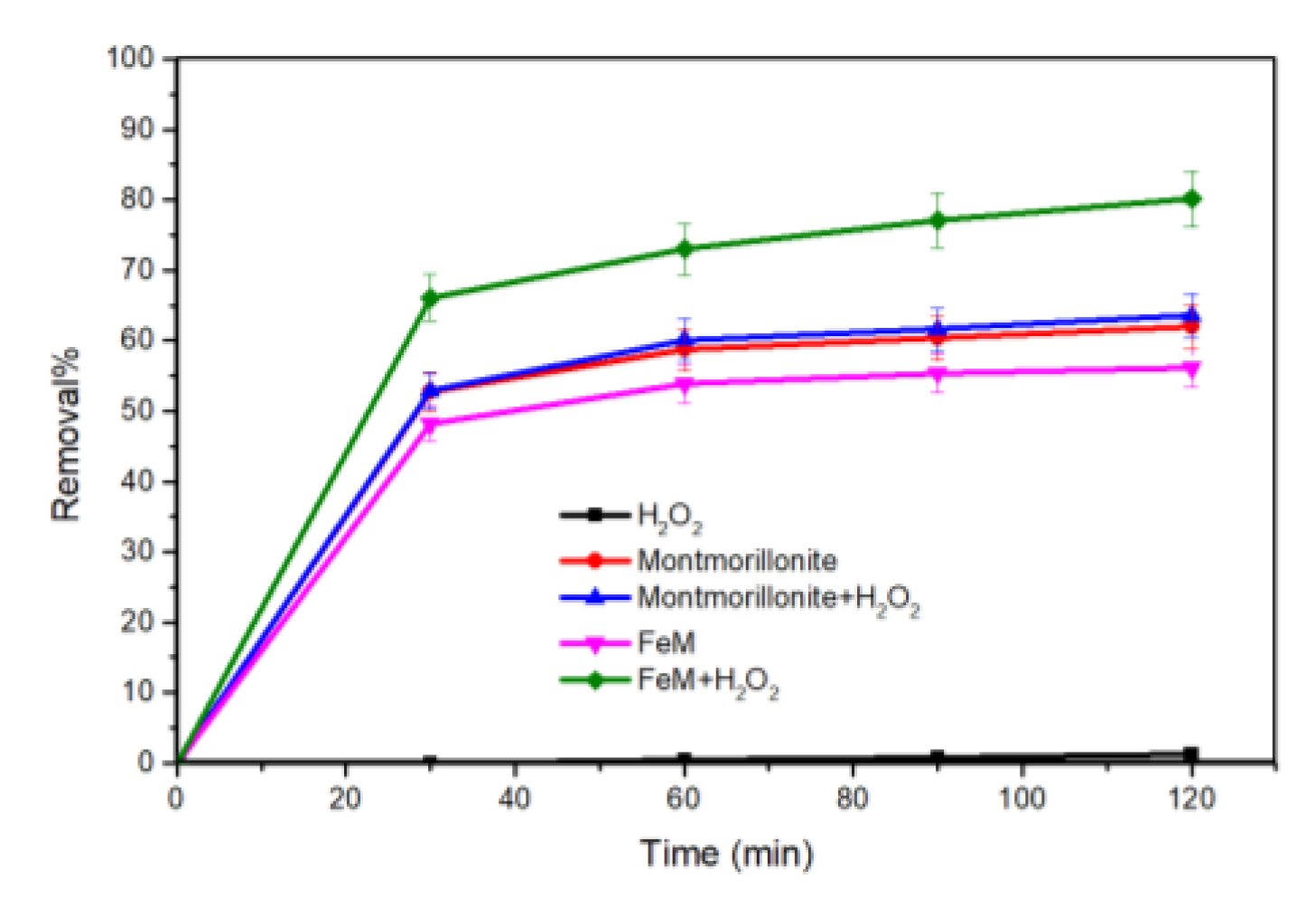
3.2.1. OFL Removal in Different Processes
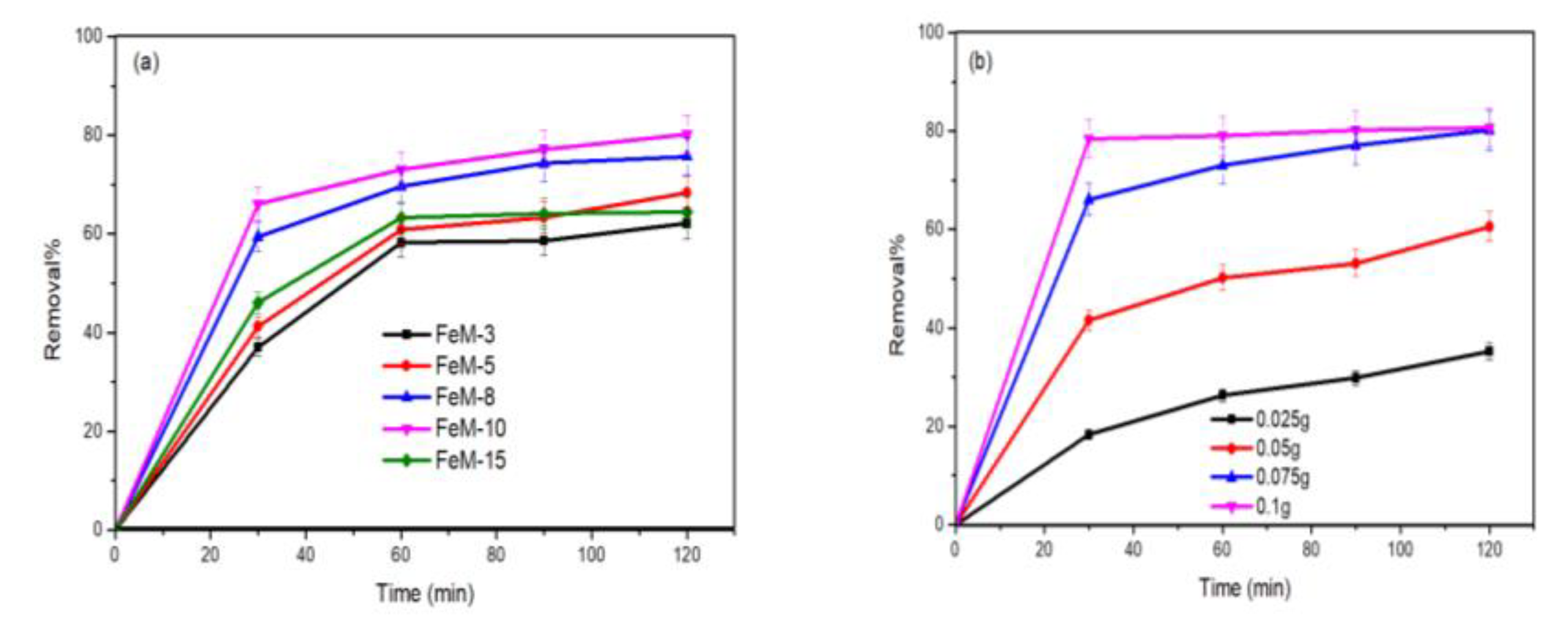
3.2.2. Influence of Process Variables
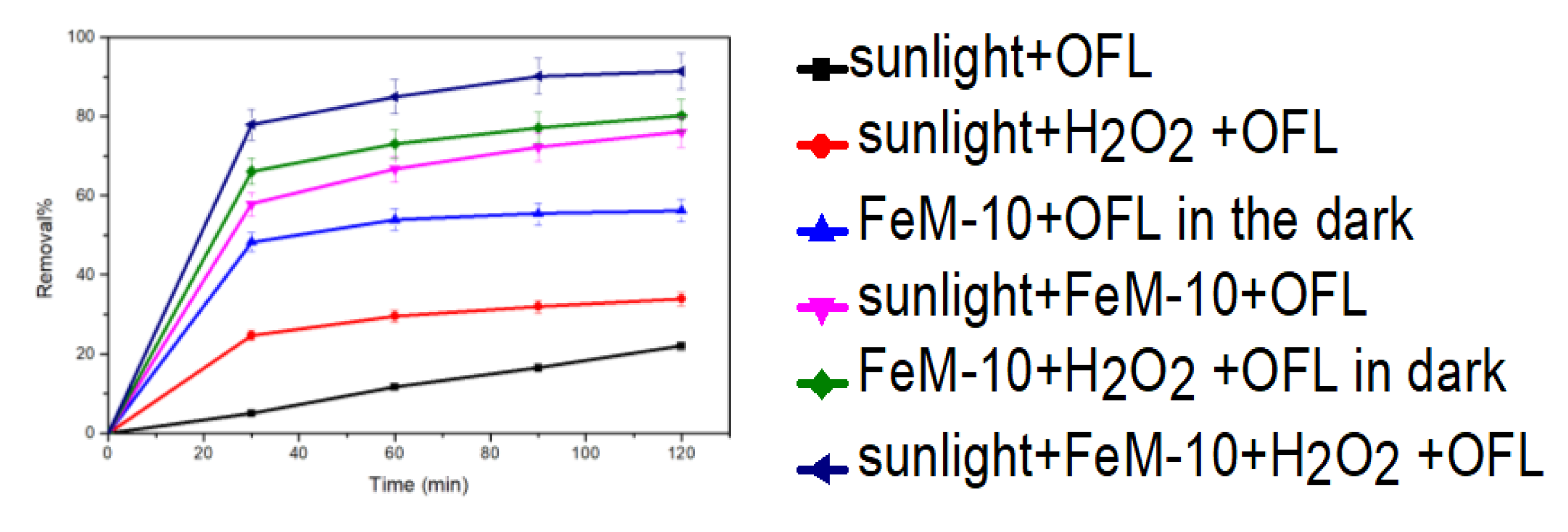
3.2.3. Photo-Fenton Catalytic Activity
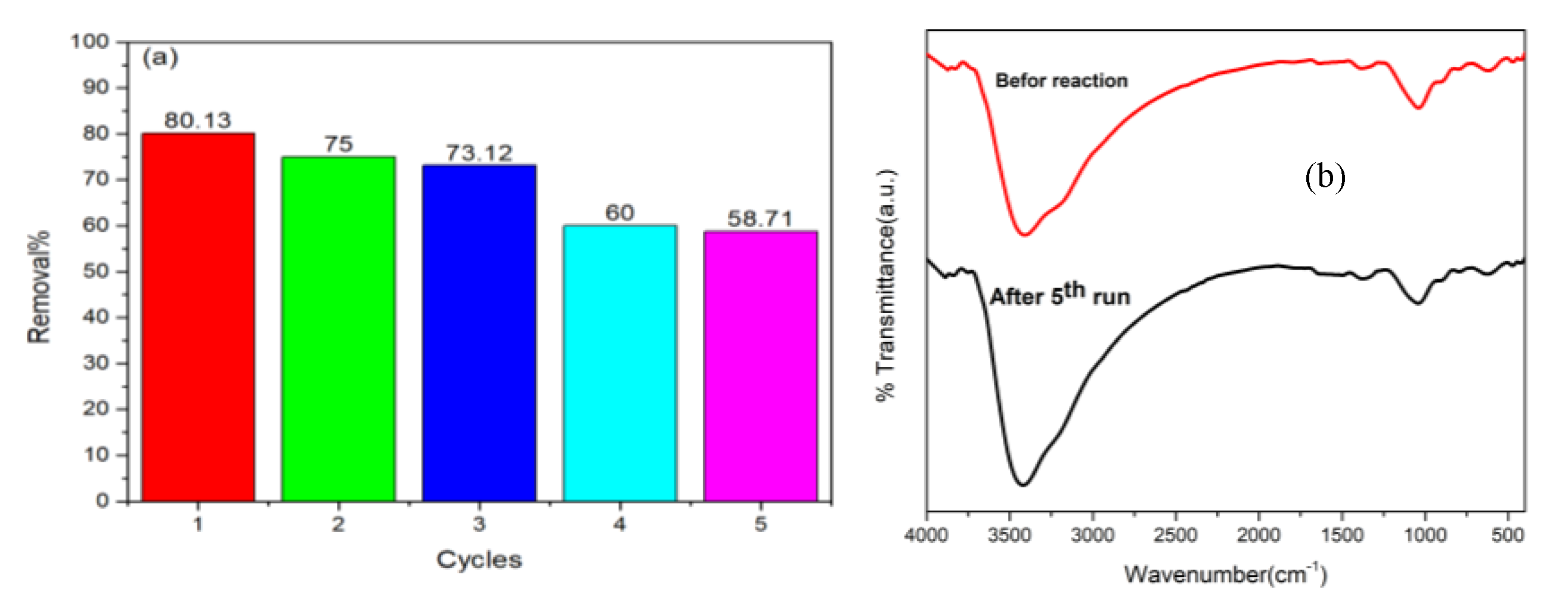
3.2.4. Reusability and Stability Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, P.; Blaney, L.; Cagnetta, G.; Huang, J.; Wang, B.; Wang, Y.; Deng, S.; Yu, G. Degradation of ofloxacin by perylene diimide supramolecular nanofiber sunlight-driven photocatalysis. Environ. Sci. Technol. 2019, 53, 1564–1575. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wu, X.; Liu, J.; Zhang, C.; Hu, Q.; Hou, X. Ofloxacin degradation by Fe3O4-CeO2/AC Fenton-like system: Optimization, kinetics, and degradation pathways. Mol. Catal. 2019, 465, 61–67. [Google Scholar] [CrossRef]
- Zhao, G.; Ding, J.; Zhou, F.; Chen, X.; Wei, L.; Gao, Q.; Wang, K.; Zhao, Q. Construction of a visible-light-driven magnetic dual Z-scheme BiVO4/g-C3N4/NiFe2O4 photocatalyst for effective removal of ofloxacin: Mechanisms and degradation pathway. Chem. Eng. J. 2020, 405, 126704. [Google Scholar] [CrossRef]
- Kaur, R.; Kushwaha, J.P.; Singh, N. Electro-catalytic oxidation of ofloxacin antibiotic in continuous reactor: Evaluation, transformation products and pathway. J. Electrochem. Soc. 2019, 166, H250. [Google Scholar] [CrossRef]
- Changotra, R.; Guin, J.P.; Khader, S.A.; Varshney, L.; Dhir, A. Electron beam induced degradation of ofloxacin in aqueous solution: Kinetics, removal mechanism and cytotoxicity assessment. Chem. Eng. J. 2019, 356, 973–984. [Google Scholar] [CrossRef]
- Tian, Y.; He, X.; Zhou, H.; Tian, X.; Nie, Y.; Zhou, Z.; Yang, C.; Li, Y. Efficient fenton-like degradation of ofloxacin over bimetallic Fe–Cu@ Sepiolite composite. Chemosphere 2020, 257, 127209. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Wang, J. Fenton-like degradation of 2, 4-dichlorophenol using Fe3O4 magnetic nanoparticles. Appl. Catal. B: Environ. 2012, 123, 117–126. [Google Scholar] [CrossRef]
- Li, L.; Lai, C.; Huang, F.; Cheng, M.; Zeng, G.; Huang, D.; Li, B.; Liu, S.; Zhang, M.; Qin, L. Degradation of naphthalene with magnetic bio-char activate hydrogen peroxide: Synergism of bio-char and Fe–Mn binary oxides. Water Res. 2019, 160, 238–248. [Google Scholar] [CrossRef]
- He, H.; Gao, C. Supraparamagnetic, conductive, and processable multifunctional graphene nanosheets coated with high-density Fe3O4 nanoparticles. ACS Appl. Mater. Interfaces 2010, 2, 3201–3210. [Google Scholar] [CrossRef]
- Hu, X.; Liu, B.; Deng, Y.; Chen, H.; Luo, S.; Sun, C.; Yang, P.; Yang, S. Adsorption and heterogeneous Fenton degradation of 17α-methyltestosterone on nano Fe3O4/MWCNTs in aqueous solution. Appl. Catal. B: Environ. 2011, 107, 274–283. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Z.; Zhou, L.; Wu, P.; Zhao, Y.; Lai, Y.; Wang, F. Heterogeneous Fenton degradation of bisphenol A using Fe3O4@ β-CD/rGO composite: Synergistic effect, principle and way of degradation. Environ. Poll. 2019, 244, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Fang, Z.; Yan, X.; Cheng, W. Heterogeneous sono-Fenton catalytic degradation of bisphenol A by Fe3O4 magnetic nanoparticles under neutral condition. Chem. Eng. J. 2012, 197, 242–249. [Google Scholar] [CrossRef]
- Wilson, D.; Langell, M. XPS analysis of oleylamine/oleic acid capped Fe3O4 nanoparticles as a function of temperature. Appl. Surface Sci. 2014, 303, 6–13. [Google Scholar] [CrossRef]
- Zhu, Y.; Tian, L.; Jiang, Z.; Pei, Y.; Xie, S.; Qiao, M.; Fan, K. Heteroepitaxial growth of gold on flowerlike magnetite: An efficacious and magnetically recyclable catalyst for chemoselective hydrogenation of crotonaldehyde to crotyl alcohol. J. Catal. 2011, 281, 106–118. [Google Scholar] [CrossRef]
- Caglar, B.; Guner, E.K.; Keles, K.; Özdokur, K.V.; Cubuk, O.; Coldur, F.; Caglar, S.; Topcu, C.; Tabak, A. Fe3O4 nanoparticles decorated smectite nanocomposite: Characterization, photocatalytic and electrocatalytic activities. Solid State Sci. 2018, 83, 122–136. [Google Scholar] [CrossRef]
- Sharma, P.; Borah, D.J.; Das, P.; Das, M.R. Cationic and anionic dye removal from aqueous solution using montmorillonite clay: Evaluation of adsorption parameters and mechanism. Desal. Water Treat. 2016, 57, 8372–8388. [Google Scholar] [CrossRef]
- Ashiq, A.; Sarkar, B.; Adassooriya, N.; Walpita, J.; Rajapaksha, A.U.; Ok, Y.S.; Vithanage, M. Sorption process of municipal solid waste biochar-montmorillonite composite for ciprofloxacin removal in aqueous media. Chemosphere 2019, 236, 124384. [Google Scholar] [CrossRef]
- Barreca, S.; Colmenares, J.J.V.; Pace, A.; Orecchio, S.; Pulgarin, C. Neutral solar photo-Fenton degradation of 4-nitrophenol on iron-enriched hybrid montmorillonite-alginate beads (Fe-MABs). J. Photochem. Photobiol. A: Chem. 2014, 282, 33–40. [Google Scholar] [CrossRef]
- Azmi, N.; Ayodele, O.; Vadivelu, V.; Asif, M.; Hameed, B. Fe-modified local clay as effective and reusable heterogeneous photo-Fenton catalyst for the decolorization of Acid Green 25. J. Taiwan Inst. Chem. Engineers 2014, 45, 1459–1467. [Google Scholar] [CrossRef]
- Wan, D.; Wang, G.; Li, W.; Wei, X. Investigation into the morphology and structure of magnetic bentonite nanocomposites with their catalytic activity. Appl. Surface Sci. 2017, 413, 398–407. [Google Scholar] [CrossRef]
- Hassan, H.; Hameed, B. Fe–clay as effective heterogeneous Fenton catalyst for the decolorization of Reactive Blue 4. Chem. Eng. J. 2011, 171, 912–918. [Google Scholar] [CrossRef]
- Khodadadi, M.; Panahi, A.H.; Al-Musawi, T.J.; Ehrampoush, M.; Mahvi, A. The catalytic activity of FeNi3@ SiO2 magnetic nanoparticles for the degradation of tetracycline in the heterogeneous Fenton-like treatment method. J. Water Process Eng. 2019, 32, 100943. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, L.; Jiang, M.; Yang, Y.; Yang, P.; Lu, J.; Ferronato, C.; Chovelon, J.-M. Ferrous-activated peroxymonosulfate oxidation of antimicrobial agent sulfaquinoxaline and structurally related compounds in aqueous solution: Kinetics, products, and transformation pathways. Environ. Sci. Pollution Res. 2017, 24, 19535–19545. [Google Scholar] [CrossRef] [PubMed]
- Tsuneda, S.; Ishihara, Y.; Hamachi, M.; Hirata, A. Inhibition effect of chlorine ion on hydroxyl radical generation in UV-H2O2 process. Water Sci. Technol. 2002, 46, 33–38. [Google Scholar] [CrossRef]
- Warang, T.; Patel, N.; Santini, A.; Bazzanella, N.; Kale, A.; Miotello, A. Pulsed laser deposition of Co3O4 nanoparticles assembled coating: Role of substrate temperature to tailor disordered to crystalline phase and related photocatalytic activity in degradation of methylene blue. Appl. Catal. A: Gen. 2012, 423, 21–27. [Google Scholar] [CrossRef]
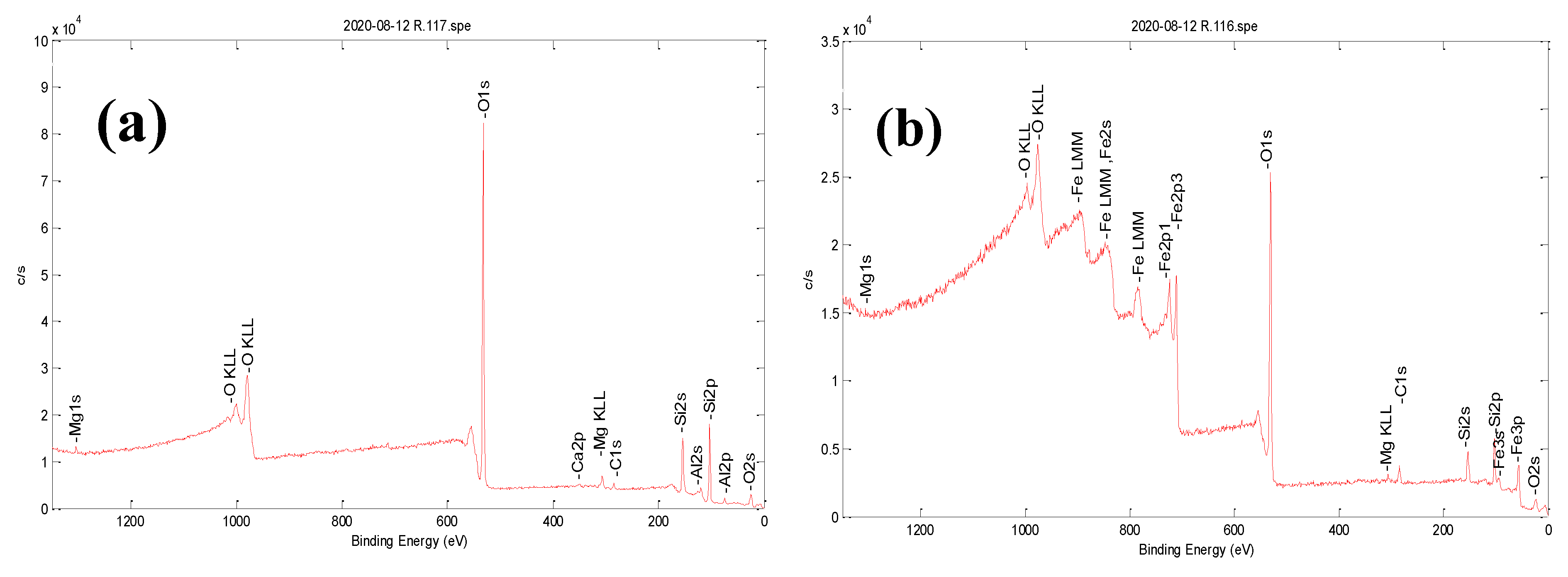
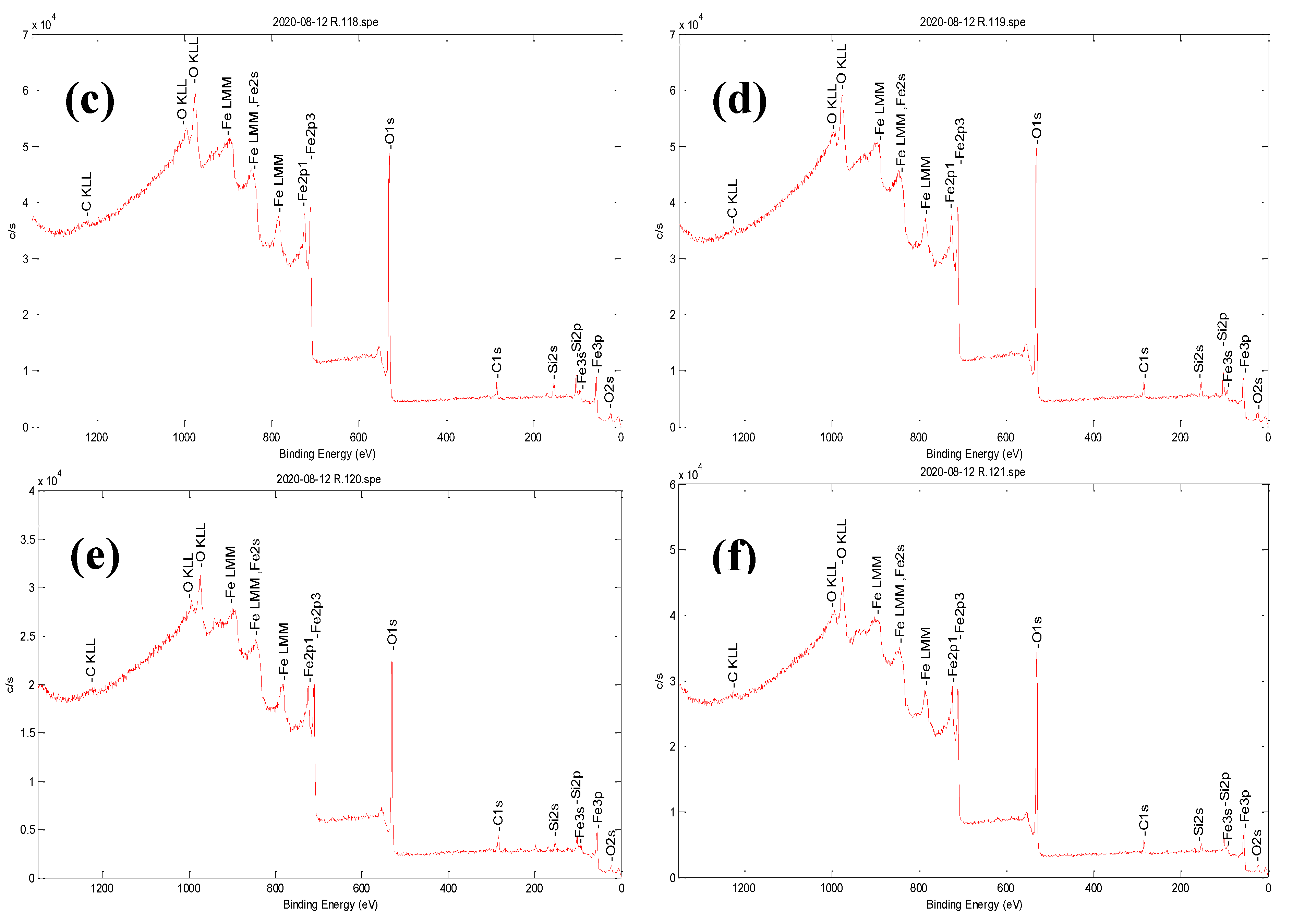

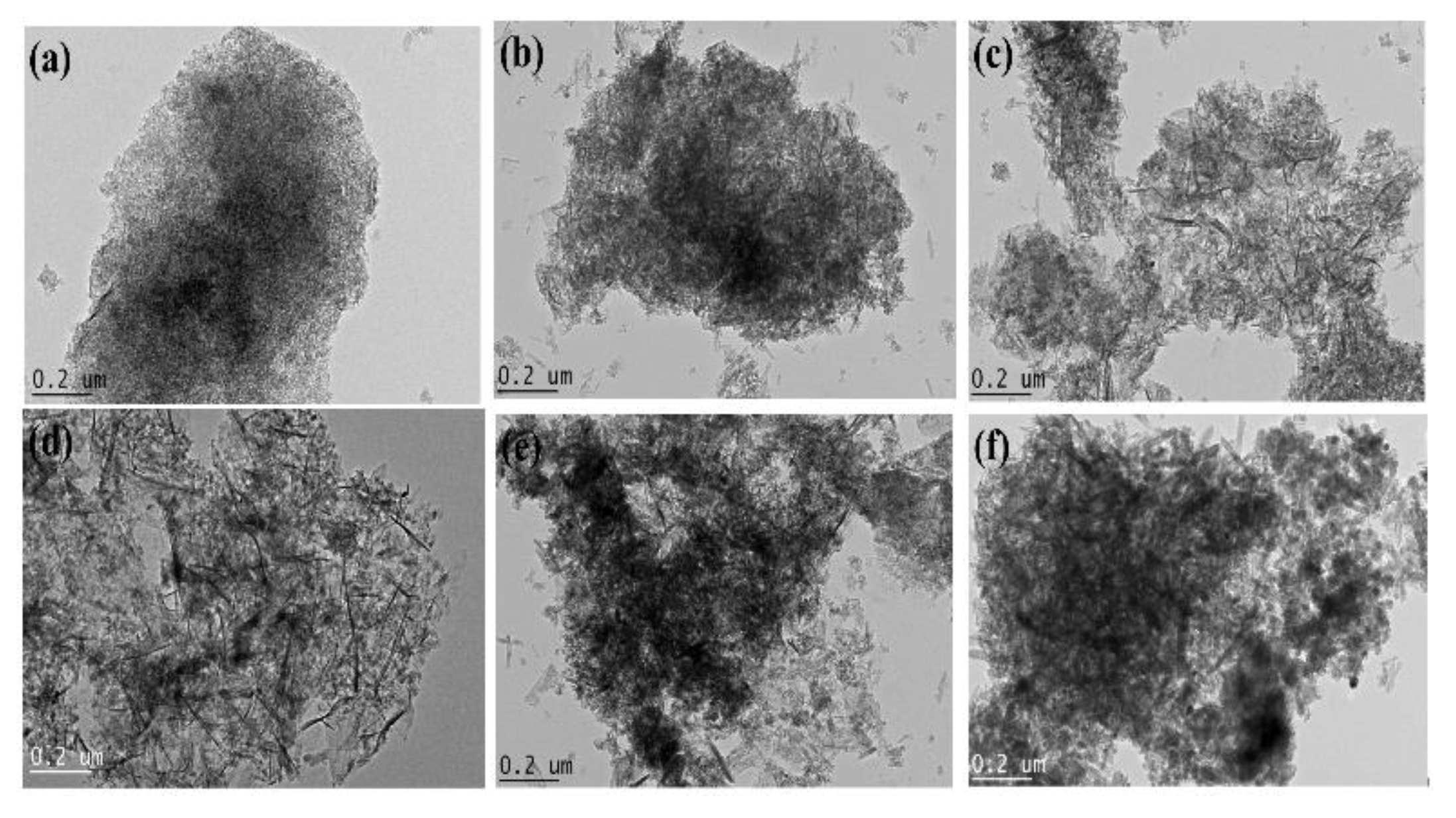
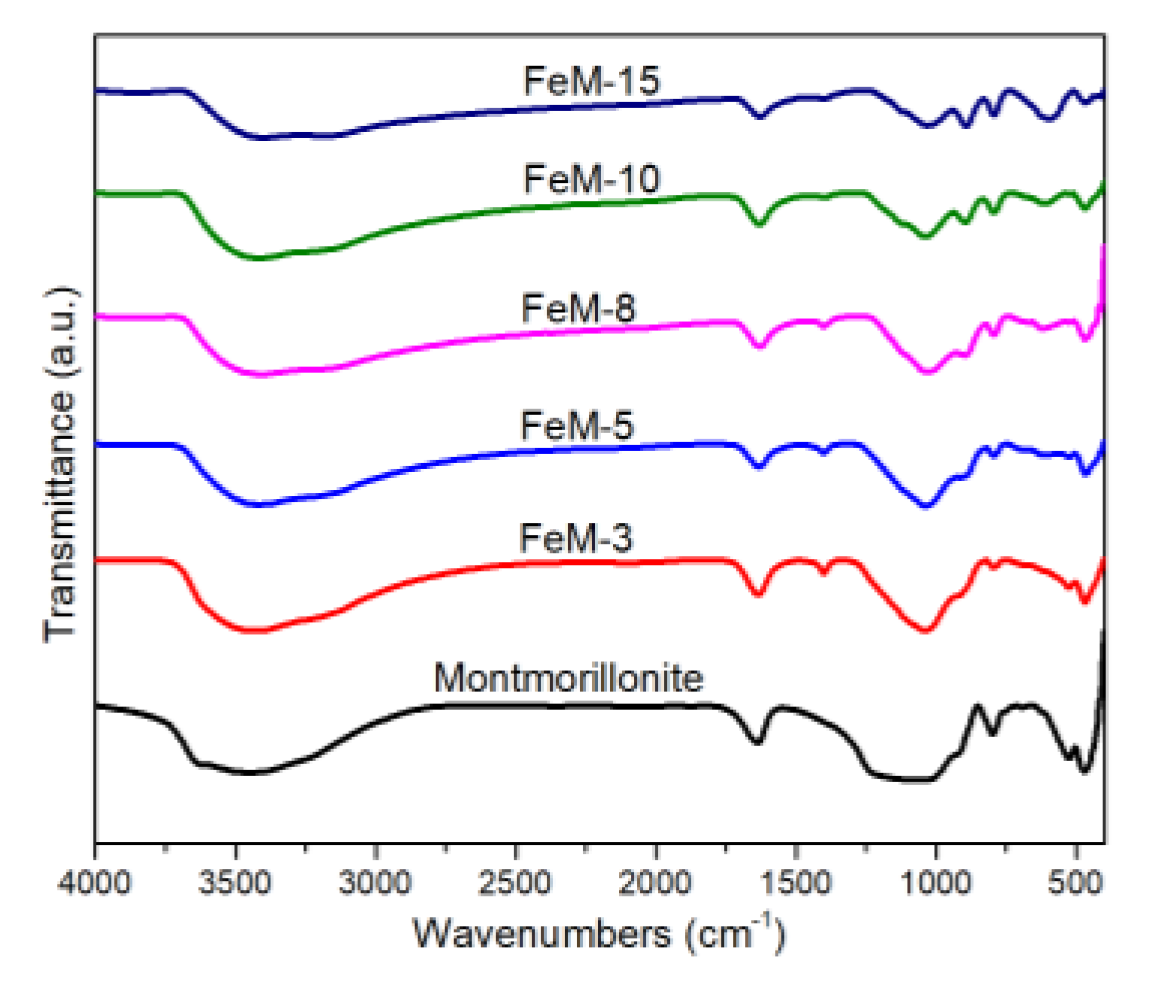


| Sample | Al % | Si % | K % | Mg% | O % | Fe % | BET Surface Area (m2/g) | Pore Volume (cm3/g) |
|---|---|---|---|---|---|---|---|---|
| Montmorillonite | 9.21 | 33.06 | 4.10 | 1.11 | 52.52 | - | 258.108 | 0.423 |
| FeM-3 | 1.61 | 17.76 | - | - | 37.36 | 43.19 | 247.944 | 0.634 |
| FeM-5 | 1.31 | 10.17 | - | - | 36.92 | 51.60 | 192.118 | 0.602 |
| FeM-8 | 0.83 | 6.58 | - | - | 33.93 | 58.66 | 163.552 | 0.384 |
| FeM-10 | 0.47 | 5.24 | - | - | 33.73 | 60.54 | 161.800 | 0.363 |
| FeM-15 | 0.18 | 2.71 | - | - | 32.96 | 64.15 | 113.186 | 0.312 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, A.R.D.; Imam, S.S.; Oh, W.D.; Adnan, R. Fenton Degradation of Ofloxacin Using a Montmorillonite-Fe3O4 Composite. Chem. Proc. 2020, 2, 32. https://doi.org/10.3390/ECCS2020-07528
Ahmad ARD, Imam SS, Oh WD, Adnan R. Fenton Degradation of Ofloxacin Using a Montmorillonite-Fe3O4 Composite. Chemistry Proceedings. 2020; 2(1):32. https://doi.org/10.3390/ECCS2020-07528
Chicago/Turabian StyleAhmad, Alamri Rahmah Dhahawi, Saifullahi Shehu Imam, Wen Da Oh, and Rohana Adnan. 2020. "Fenton Degradation of Ofloxacin Using a Montmorillonite-Fe3O4 Composite" Chemistry Proceedings 2, no. 1: 32. https://doi.org/10.3390/ECCS2020-07528
APA StyleAhmad, A. R. D., Imam, S. S., Oh, W. D., & Adnan, R. (2020). Fenton Degradation of Ofloxacin Using a Montmorillonite-Fe3O4 Composite. Chemistry Proceedings, 2(1), 32. https://doi.org/10.3390/ECCS2020-07528






