New High-Throughput Reactor for Biomass Valorization †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preapration of Heterogeneous Catalyst: Calcium Diglyceroxide
2.2. Heterogeneoous Transesterification Reaction with Methanol
3. Results
3.1. Synthesis Optimization of Calcium Diglyceroxide
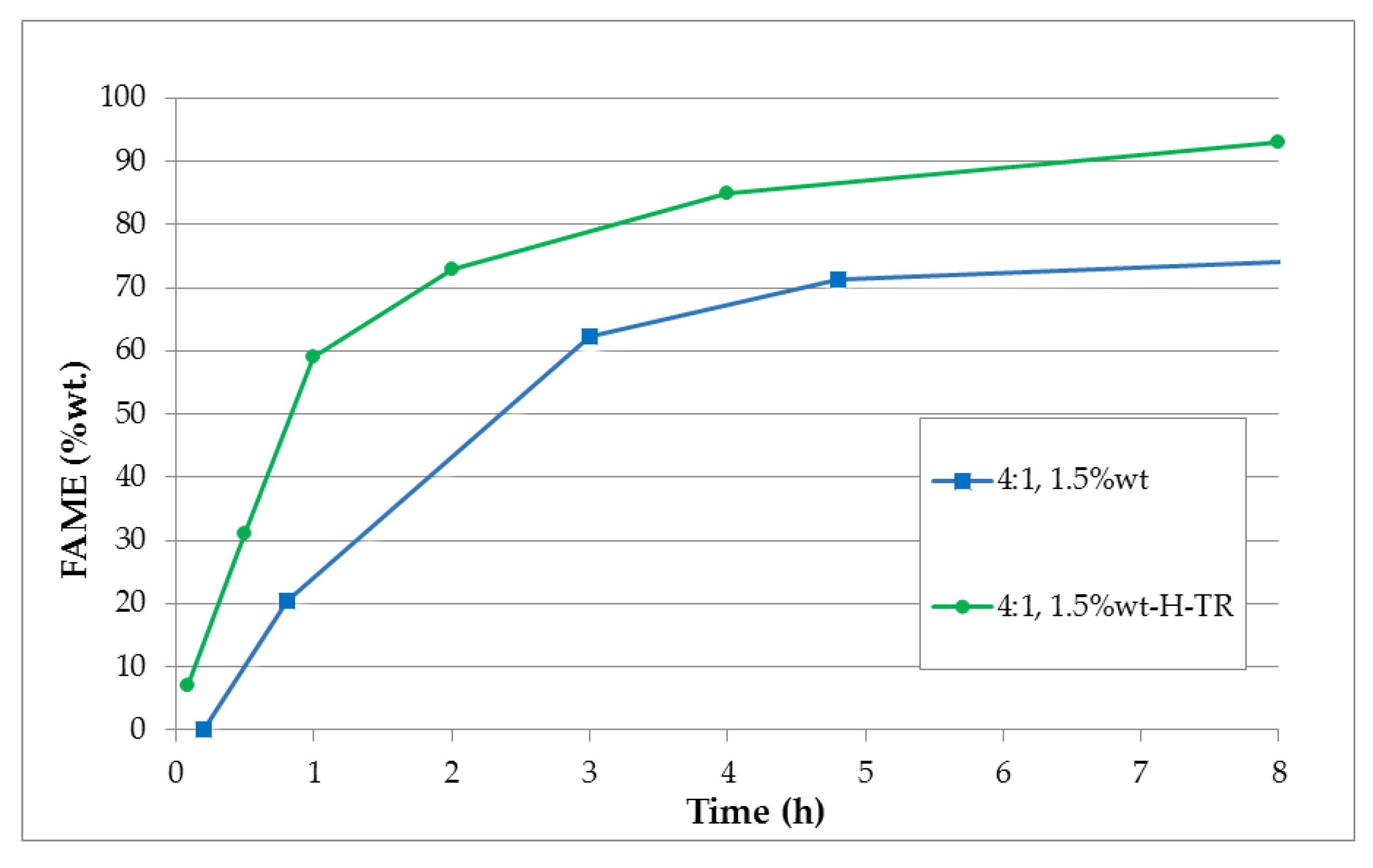
3.2. Heterogeneoous Transesterification Reaction with Methanol
4. Conclusions
5. Patents
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Rodríguez-Padrón, D.; Puente-Santiago, A.; Balu, A.; Muñoz-Batista, M.; Luque, R. Environmental catalysis: Present and future. ChemCatChem 2019, 11, 18–38. [Google Scholar] [CrossRef]
- Kurosawa, W.; Nakano, T.; Amino, Y. Practical large-scale production of dihydrocapsiate, a nonpungent capsaicinoid-like substance. Biosci. Biotechnol. Biochem. 2017, 81, 211. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Batista, M.J.; Rodríguez-Padrón, D.; Puente-Santiago, A.; Luque, R. Mechanochemistry: Toward sustainable design of advanced nanomaterials for electrochemical energy storage and catalytic applications. ACS Sustain. Chem. Eng. 2018, 8, 9530–9544. [Google Scholar] [CrossRef]
- The European Green Deal. Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions; European Commission Communications: Brussels, Belgium, 2019. [Google Scholar]
- Schöppe, H.; Kleine-Möllhoff, P.; Epple, R. Energy and material flows and carbon footprint assessment concerning the production of HMF and furfural from a cellulosic biomass. Processes 2020, 8, 119. [Google Scholar] [CrossRef]
- Gui, M.M.; Lee, K.T.; Bhatia, S. Feasibility of edible oil vs. non-edible oil vs. waste edible oil as biodiesel feedstock. Energy 2008, 33, 1646–1653. [Google Scholar] [CrossRef]
- Bankovic-Ilic, I.B.; Stamenkovic, O.S.; Veljkovic, V.B. Biodiesel production from non-edible plant oils. Renew. Sustain. Energy Rev. 2012, 16, 3621–3647. [Google Scholar] [CrossRef]
- Lee, A.F.; Wilson, K. Recent developments in heterogeneous catalysis for the sustainable production of biodiesel. Catal. Today 2015, 242, 3–18. [Google Scholar] [CrossRef]
- Kouzu, M.; Fujimori, A.; ta Fukakusa, R.; Satomi, N.; Yahagi, S. Continuous production of biodiesel by the CaO-catalyzed transesterification operated with continuously stirred tank reactor. Fuel Process. Technol. 2018, 181, 311–317. [Google Scholar] [CrossRef]
- Kouzu, M.; Kasuno, T.; Tajika, M.; Yamanaka, S.; Hidaka, J. Active phase of calcium oxide used as solid base catalyst for transesterification of soybean oil with refluxing methanol. Appl. Catal. Gen. 2008, 334, 357–365. [Google Scholar] [CrossRef]
- Buasri, A.; Lukkanasiri, M.; Nernrimnong, R.; Tonseeya, S.; Rochanakit, K.; Wongvitvichot, W.; Masa-ard, U.; Loryuenyong, V. Rapid transesterification of Jatropha curcas oil to biodiesel using novel catalyst with a microwave heating system. Korean J. Chem. Eng. 2016, 33, 3388–3400. [Google Scholar] [CrossRef]
- Lukic, I.; Kesic, Z.; Zdujic, M.; Skala, D. Calcium diglyceroxide synthesized by mechanochemical treatment, its characterization and application as catalyst for fatty acid methyl esters production. Fuel 2016, 165, 159–165. [Google Scholar] [CrossRef]
- Lacoste, F.; Thiel, J.; Lair, V.; Halloumi, S. Method for Manufacturing Calcium Diglyceroxide. Patent WO 2018/060654 A1, 5 April 2018. [Google Scholar]
- Kouzu, M.; Hidaka, J.S. Transesterification of vegetable oil into biodiesel catalyzed by CaO: A review. Fuel 2012, 93, 1–12. [Google Scholar] [CrossRef]
- Reyero, I.; Arzamendi, G.; Gandía, L.M. Heterogenization of the biodiesel synthesis catalysis: CaO and novel calcium compounds as transesterification catalysts. Chem. Eng. Res. Des. 2014, 92, 1519–1530. [Google Scholar] [CrossRef]
- Xiao, Y.; Gao, L.; Xiao, G.; Fu, B.; Niu, L. Experimental and modeling study of continuous catalytic transesterification to biodiesel in a bench-scale fixed-bed reactor. Ind. Eng. Chem. Res. 2012, 51, 11860–11865. [Google Scholar] [CrossRef]
- Lacoste, F.; Thiel, J.; Lair, V.; Halloumi, S. Method for Producing Fatty Acid Esters and Glycerol at Low. Temperature. Patent WO 2018/002559 A1, 4 January 2018. [Google Scholar]
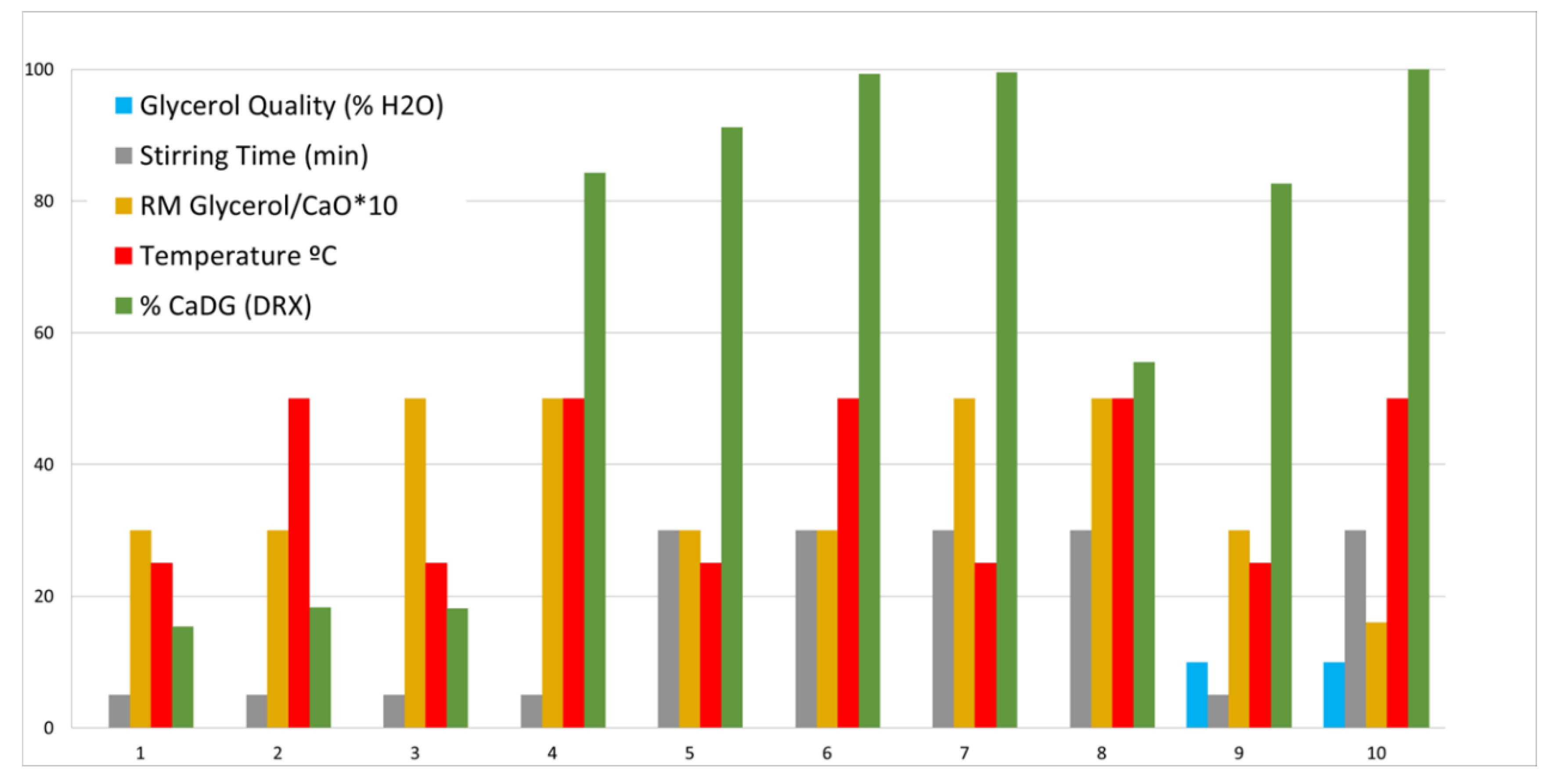
| Entry | Glycerol Quality (wt.% H2O) | Stirring Time (min) | Glyc/CaO MR * | Temperature (°C) | % CaDG (XRD) |
|---|---|---|---|---|---|
| 1 | 0 | 5 | 30 | 25 | 15.40 |
| 2 | 0 | 5 | 30 | 50 | 18.30 |
| 3 | 0 | 5 | 50 | 25 | 18.10 |
| 4 | 0 | 5 | 50 | 50 | 84.30 |
| 5 | 0 | 30 | 30 | 25 | 91.20 |
| 6 | 0 | 30 | 30 | 50 | 99.30 |
| 7 | 0 | 30 | 50 | 25 | 99.50 |
| 8 | 0 | 30 | 50 | 50 | 55.50 |
| 9 | 10 | 5 | 30 | 25 | 82.60 |
| 10 | 10 | 30 | 50 | 50 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malpartida, I.; Maireles-Torres, P.; Lair, V.; Halloumi, S.; Thiel, J.; Lacoste, F. New High-Throughput Reactor for Biomass Valorization. Chem. Proc. 2020, 2, 31. https://doi.org/10.3390/ECCS2020-07583
Malpartida I, Maireles-Torres P, Lair V, Halloumi S, Thiel J, Lacoste F. New High-Throughput Reactor for Biomass Valorization. Chemistry Proceedings. 2020; 2(1):31. https://doi.org/10.3390/ECCS2020-07583
Chicago/Turabian StyleMalpartida, Irene, Pedro Maireles-Torres, Valentin Lair, Samy Halloumi, Julien Thiel, and François Lacoste. 2020. "New High-Throughput Reactor for Biomass Valorization" Chemistry Proceedings 2, no. 1: 31. https://doi.org/10.3390/ECCS2020-07583
APA StyleMalpartida, I., Maireles-Torres, P., Lair, V., Halloumi, S., Thiel, J., & Lacoste, F. (2020). New High-Throughput Reactor for Biomass Valorization. Chemistry Proceedings, 2(1), 31. https://doi.org/10.3390/ECCS2020-07583






