3. Results and Discussion
Commercial ZnO with a low surface area was subjected to milling. When WC was used, an increase in accumulated kinetic energy (E
acum) was observed with an increase in the milling time [
1]. Along the same lines, the S
BET also increased until 60 min of milling (6.03 m
2 g
−1), decreasing later due to a process of agglomeration of the particles. To determine the effect of the use of ZrO
2 on the support, the E
acum of the WC at 60 min of milling was taken as a reference, and the time necessary to reach it was calculated, which was only 6 min. Milled ZnO presented a S
BET three times higher than that of the unmilled support (
Table 1); therefore, it was selected as a support for the Cux/ZnO-z system. In order to determine the effect of milling on the metal-support interaction, the Cux/ZnO-0 system was synthesized as a reference. The characterization of the supports is not presented due to the space limit.
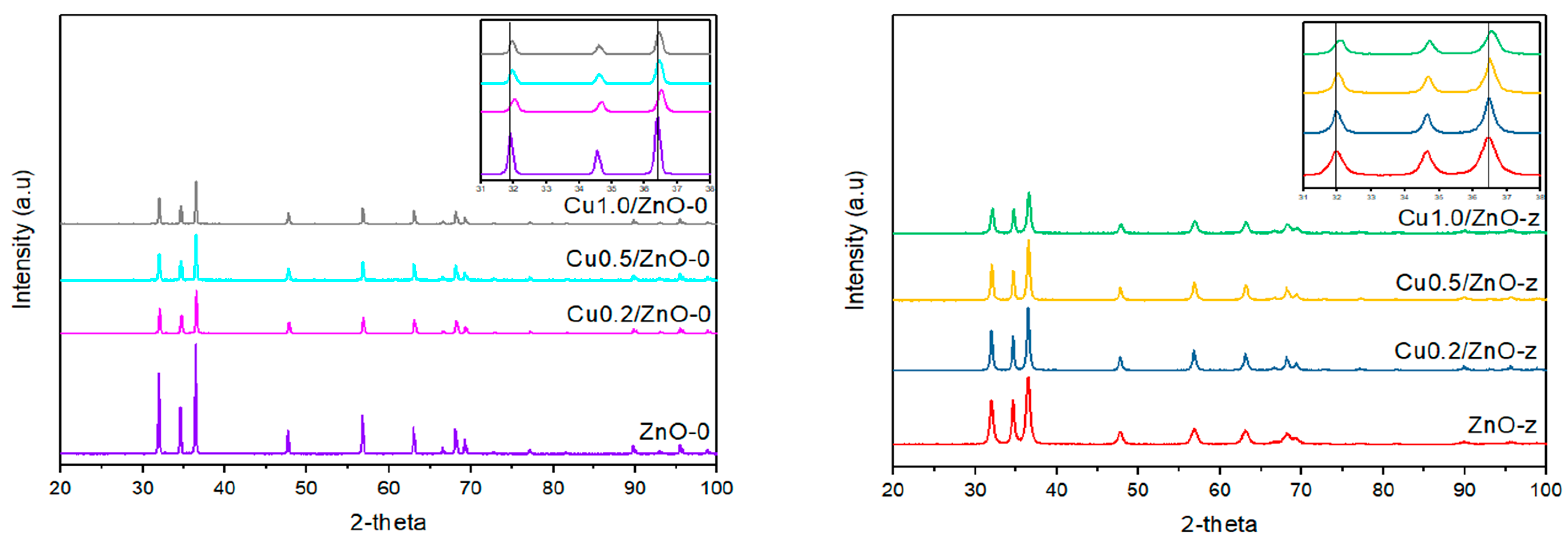
XRD diffractograms are shown in
Figure 1. In both systems, diffraction lines typical of the hexagonal system of wurtzite (PDF 96-90-4181) can be observed, without any diffraction lines corresponding to phases containing Cu. This may be due to its low Cu concentration, high dispersion and/or the incorporation of Cu ions in the ZnO structure. The observed shift of the most intense diffraction signal (101) of ZnO (
Figure 1) indicates the incorporation of Cu into the ZnO lattice, in different extensions, in both systems. In the Cux/ZnO-0 system, the increase of the Cu content leads to a decrease in the displacement. Cu0.2/ZnO-0 shows the highest displacement, which is a sign of constant lattice contraction. This can be attributed to the internal relaxation of the lattice, due to the incorporation of Cu
2+ ions (0.073 nm) in the bigger Zn sites (0.074 nm) [
4]. For 0.5% and 1.0%, the lower displacements can be associated with a lower incorporation of Cu
2+ in the ZnO lattice, and a favouring of the nucleation process and the subsequent growth rate of CuO, due to the higher concentration of Cu. Contrary to Cux/ZnO-z, the diffraction lines shift towards greater angles with increasing Cu content. This can be associated with the continuous migration of the Cu
2+ species in the ZnO matrix, occupying positions in its planar defects, whose content is higher in ZnO-z due to milling.
Different parameters of the ZnO crystal structure, for both systems, calculated from the XRD data, Scherrer equation and Rietveld modelling are presented in
Table 1 [
4]. For Cux/ZnO-0 it can be observed that the D values are lower than those with the support, which can be associated with the alteration in the host lattice, due to the insertion of Cu. However, D increases with the increase in Cu content, indicating a lower insertion of Cu
2+ in the ZnO lattice, in line with what was observed by XRD. The ZnO-z support has a D lower than ZnO-0, as a consequence of the milling. In Cux/ZnO-z, an increase in D is observed when Cu is added, which would indicate the incorporation of Cu in the ZnO. However, D decreases when more Cu is added. Although with the increase in copper, more Cu
2+ ions are incorporated, it is possible that they are located in positions that stabilize the ZnO-z lattice and/or part of the Cu
2+ located in tetrahedral positions is reduced to Cu
+, which has a lower ionic radius (0.060 nm) and leads to external relaxation of the closest O atoms, contracting the crystalline lattice. It has been reported that Cu
+ ions can come from the transformation of Cu
2+ ions that capture an extra electron from a nearby atom or in complexes with oxygen vacancies [
5].
For both systems, the c/a cell parameter relationship changes concerning the supports, which indicates a structural deformation of the material [
4], confirming the incorporation of Cu in it. For Cux/ZnO-0 the c/a ratio decreases, while no variations are observed when the Cu doping increases, which can be explained by a limited insertion of the cation in the support lattice, for the three catalysts. In Cux/ZnO-z, the c/a ratio is higher than that of the support and it increases with the addition of Cu, due to its continuous incorporation into the structure. The intrinsic stress (ξ) and the density of the grain boundaries (dislocations (δ)) also provide information on stresses and defects introduced in the support crystalline lattice by the incorporation of an ion and/or milling [
4]. In
Table 1, it can be observed that for the Cux/ZnO-0 system, the ξ and δ values increase in the support, which can be attributed to the changes in the microstructure, size, and shape of the particles, due to the Cu insertion. However, ξ and δ decrease with the increase of Cu, which would indicate that the nucleation process of the Cu excess is favoured, instead its incorporation into the structure. As expected for the support subjected to milling, ξ and δ present high values due to the great stresses and defects introduced in the lattice. In the Cux/ZnO-z system, with the addition of Cu, ξ and δ tend to decrease, which would indicate that the Cu atoms could occupy positions in the lattice, stabilizing it and releasing the stress generated by the milling. However, with the increase in Cu content, both increase due to a greater incorporation of Cu, without reaching the values obtained for ZnO-z. Therefore, it could be said that doping with Cu, in general, improves the stability of the lattice.
Raman spectroscopy corroborates what is mentioned above. In the supports, the E2(high) no polar mode predominates at ~438 cm
−1, which it is characteristic of the wurtzite hexagonal phase. The intensity of this mode dramatically decreases with the milling. Furthermore, it expands and increases its asymmetry, indicating a further disarray of the lattice. The absence of vibrational modes corresponding to the Cu oxide phases demonstrates its incorporation into the ZnO lattice and/or a high dispersion of CuO. It has been reported that the I
E1/I
2A1 intensities the ratio, allowing the identification of the content of defects in a solid, in particular, the oxygen vacancy type [
6]. For Cux/ZnO-0 this ratio is higher than for the support, but it decreases with the Cu content increase. However, for Cux/ZnO-z, this ratio decreases considering the support, indicating a decrease in the number of defects and the Cu content increase.
The S
BET of the Cux/ZnO-0 system practically does not vary with the doping of Cu. On the contrary, in the Cux/ZnO-z system, the S
BET decreases with the incorporation of Cu, but increases with the increase of the Cu content. This could be associated with the impregnation stage, where the incorporated moisture and the subsequent calcination would favour the coalition of the ZnO small agglomerated particles, obtained during the milling. The impregnating solution of copper acetate with a pH = 5.5–6.0 could erode the ZnO surface, which is easily attacked by acids, explaining the slight increase in the S
BET with the increase in the Cu content, in both systems. The composition data, obtained by atomic absorption for both families, show excellent impregnation, since the experimental values are similar to the theoretical ones (
Table 1).
XPS was used to understand the surface modifications introduced by the milling on the support, and the interaction achieved between the metal-support (
Table 2). In both systems, shifts were observed in the Zn 2p1/2 and 2p3/2 signals, corresponding to ZnO [
7] at higher binding energies (BE), with respect to the two supports, indicating a strong Cu-ZnO interaction. In Cux/ZnO-0 the displacement decreases with increasing Cu content, which would indicate a decrease in this interaction as more Cu is deposited. An inverse behaviour can be observed in Cux/ZnO-z, indicating a greater Cu-ZnO interaction as more metal is deposited. Cu 2p1/2 and 2p3/2 signals also show shifts, but more marked for the two systems, but at lower BE than those reported in the literature for CuO (953.2 eV and 933.5 eV) [
7]. This is associated with the Cu-ZnO interaction. With increasing copper content, there is no linear behaviour. According to I. Kaskow et al. [
7], the increase in the BE in Zn 2p3/2, and its decrease in the supported metal, implies an electronic transfer from the support to the metal. This could induce the reduction of CuO to Cu
2O, at the interface between the metal domain and the support, in both families. The presence of a satellite signal at ~943 eV is characteristic of materials having a d9 configuration in its highest oxidation state (CuO). In Cux/ZnO-0, this satellite is almost imperceptible for 0.2%, but its intensity increases with the Cu content. For Cux/ZnO-z, it can only be observed with a very low intensity (1.0%). Although the CuO formation increases with the addition of Cu, in both systems, its content is higher in the Cux/ZnO-0 system. The O1s signal was deconvolved into two signals (
Table 2). These signals correspond to oxygen of the crystalline lattice, O
lat, at 530.1–530.4 eV, and oxygen adsorbed in oxygen vacancies, O
ads, at 531.7–532.3 eV. Milling causes a shift of these signals to lower BE, which is associated with internal stresses in the lattice, and generated defects. For Cux/ZnO-0, the O
lat do not vary with the Cu content increase, but their signal moves to higher BE. This could be explained by the limited incorporation of the Cu in the ZnO lattice. Contrary to O
ads, its BE increases with the copper content, being higher than that of the support, which could indicate a strong interaction of Cu with these defects. Indeed, this is the case, since the total content of O
ads decreases with the addition of Cu, which would indicate a selective adsorption of the metal in this type of defect, in line with the variations in the ratio of intensities observed by Raman. In Cux/ZnO-z, the O
lat moves to higher BE and increases with the addition of copper. This can be associated with the constant incorporation of Cu into ZnO-z. The O
ads shows a lower displacement at a higher EB with respect to the support, but it also increases with the Cu content. In this system, copper is also selectively adsorbed on oxygen vacancies, but to a lesser degree than for the Cux/ZnO-0 system. If we compare the occupied O
ads in percentage terms, after Cu deposition, for Cu1.0/ZnO-0 it is 51%, while for Cu1.0/ZnO-z it is 30%, and at the other extreme, for Cu0.2/ZnO-z, the O
ads are not modified by the addition of Cu, while for Cu0.2/ZnO-0 30% is occupied. These results allow us to infer that in Cux/ZnO-0 part of the deposited Cu is incorporated into the ZnO lattice, while the rest of it is selectively adsorbed on the oxygen vacancies, leading to a nucleation process to obtain CuO. As in Cux/ZnO-z, a higher percentage of Cu is incorporated into the lattice of the milled support. The rest is located in the oxygen vacancies, forming CuO. Therefore, different Cu contents with Cu
2+ and Cu
+ valences can be expected in the two systems.
RTP is a bulk technique that provides information on the reducibility of the systems, the possible Cu oxidation states, and Cu-ZnO interaction. The reduction profiles of the catalysts in both systems are the same. They present two overlapped signals, which move to lower reduction temperatures with the Cu content increase, while the supports do not present reduction signals in the temperature range studied. For Cux/ZnO-z the copper reducibility is higher than in the other system. All the catalysts have lower Cu reduction temperatures than those corresponding to the bulk CuO reduction (347 °C), indicating a strong copper-support interaction. The two signals present in the reduction profiles have been associated with the reduction of different copper species by different authors. Gao et al. [
2] associate the first signal to the reduction of highly dispersed CuO and the signal at high temperature to bulk CuO. G. Fierro et al. [
8] associate them with the reduction of CuO with different interactions with ZnO, while X. Yang et al. [
3] associate the signal at low temperature to the reduction of Cu
2+ to Cu
+ and the signal at high temperature to the reduction of Cu
+ to Cu°. With the aim to better understand our results, the H
2 consumptions were calculated and compared with the theoretical values, corresponding to the Cu content deposited in each catalyst for the reduction of CuO and Cu
2O (
Table 2). Cux/ZnO-0 presents consumptions close to the theoretical ones for the exclusive reduction of CuO, but when the copper content increases, the experimental consumptions are lower than the theoretical ones, which could indicate the presence of a low amount of Cu
2O. The coexistence of copper with both valences is possible due to electronic mobility that occurs from the support to the metal, as inferred from XPS. The greater amount of Cu
2O with the increase in the copper content could be associated with highly dispersed small metallic domains, where the bulk of the metallic particle corresponds to CuO, and the metal-support contact area, which would increase with dispersion and domain size reduction, is where Cu
2O would be formed. On the contrary, Cux/ZnO-z presents experimental consumptions close to the theoretical consumptions for the exclusive reduction of Cu
2O. In this case, with the increase in the copper content, the theoretical consumptions are higher than the theoretical ones for the exclusive reduction of Cu
2O, which would indicate the presence of a small amount of CuO. The possibility of having Cu
2O and CuO, but in an inverse relationship to that of the unmilled system, could be explained by the greater incorporation of Cu in the ZnO lattice. This facilitates its reduction, possibly due to the formation of very small metallic domains, dispersed and with a greater interaction with the support, at low bulk CuO, but increasing when more copper is added.