Abstract
In this work, Pt-Ni/CeO2-SiO2, as well as Ru-Ni/CeO2-SiO2 catalysts, were obtained at different loadings of the noble metal (in the interval 0–3 wt%) and tested for oxidative steam reforming of ethanol. Stability performance was evaluated at 500 °C for 25 h under a steam to ethanol ratio of 4 and an oxygen to ethanol ratio of 0.5. The weight hourly space velocity was fixed to 60 h−1, which is considerably higher than the typical values selected for such processes. All the catalysts deactivated with time-on-stream, due to the severe operative conditions selected. However, the highest ethanol conversion (above 95%) and hydrogen yield (30%) at the end of the test were recorded over the 2 wt%Pt-10 wt%Ni/CeO2-SiO2 catalyst, which also displayed a limited carbon formation rate (1.5 × 10−6 gcoke·gcatalyst−1·gcarbon,fed−1·h−1, reduced almost 5 times compared to the samples that had a Pt or Ru content of 0.5 wt%). Thus, the latter catalyst was identified as a promising candidate for future tests under real bioethanol mixture.
1. Introduction
The development of sustainable processes for energy generation is one of the most important goals of academic as well as industrial research. In this field, hydrogen conversion in fuel cells is gaining increasing attention—water is the only byproduct and any other pollutant (i.e., CO2) is released [1,2]. However, at present, hydrogen production predominantly depends on fossil fuels resources, with methane steam reforming being the most widespread method for H2 generation and nearly half of the world’s hydrogen still deriving from natural gas [3]. Thus, in response to the problems of fossil fuels depletion and environmental pollution, the methods of producing hydrogen from biomass have been regarded as potential ways to ensure hydrogen becomes the fuel of the future. To this end, the catalytic reforming of bioethanol (produced by fermentation of biomass sources) has been proposed as an interesting alternative [4,5].
However, the reaction mechanism is quite complex and several reactions may occur, reducing hydrogen yield and causing carbon deposition on the catalyst’s surface. Thus, the development of active catalysts with high resistance towards deactivation is one of the main issues in this process. To this end, oxygen co-feeding was reported as a useful tool to promote the oxidation of carbonaceous deposits.
The performance of a large number of catalysts for ethanol reforming has been investigated in the literature—the combination of nickel with reduced amounts of noble metals was proposed to mitigate the coke formation tendency of nickel and limit and, at the same time, the price of the final catalyst [6,7,8]. The choice of reducible support with a high surface area was also reported to improve the catalyst’s resistance towards deactivation [9]. In our previous work, highly dispersed 3 wt%Pt-10 wt%Ni/CeO2-SiO2 catalysts have been prepared, which displayed high stability during oxidative steam reforming of ethanol [10].
In the present work, the effect of Pt loading on catalyst resistance towards deactivation was investigated and the chance of substituting Pt by a less expensive metal (i.e., ruthenium) was also discussed. The catalysts were tested under a simulated bioethanol stream (Ethanol:Water = 1:4) by fixing a contact time of 50 ms, much lower than the typical values selected for reforming reactions. The time on stream tests was performed for 25 h and the performance of the catalysts that had various metal loadings were compared in terms of ethanol conversion, hydrogen yield and carbon formation rate.
2. Materials and Methods
The catalysts were prepared by the sequential impregnation of the non-noble metal and noble metal on the CeO2-SiO2 support. The latter material was prepared by adding calcined mesoporous silica gel (provided by Sigma-Aldrich) to a solution of acetic acid/methanol (40/60 % vol.) containing the ceria salt precursor (i.e., Cerium acetylacetonate). Nickel was deposited via the impregnation of CeO2-SiO2 with an aqueous solution of nickel nitrate hexahydrate, while platinum chloride and ruthenium chloride were selected as precursors of the noble metals. The CeO2/SiO2 ratio was fixed to 0.3 while nickel content with respect to the ceria mass was equal to 10 wt%; the loading of the noble metal was changed in the interval 0–3 wt%. Thus, the following catalysts were prepared: Ni/CeO2-SiO2, 0.5Pt-Ni/CeO2-SiO2, 1Pt-Ni/CeO2-SiO2, 2Pt-Ni/CeO2-SiO2, 3Pt-Ni/CeO2-SiO2, 0.5Ru -Ni/CeO2-SiO2, 1Ru -Ni/CeO2-SiO2, 2Ru-Ni/CeO2-SiO2, 3Ru-Ni/CeO2-SiO2.
The specific surface area of fresh and spent catalysts was determined via the BET (Brunauer–Emmett–Teller) method by N2 adsorption at −196 °C (Costech Sorptometer 1040); before the analysis, the samples were degassed at 150 °C under vacuum.
Temperature programmed reduction measurements (TPR) were performed in the laboratory apparatus described below. The temperature was raised to 700 °C under a 5%H2/Ar stream and hydrogen consumption was estimated from the profiles acquired via a Mass Spectrometer (supplied by Hiden Analytical).
The carbon content on the spent catalyst was measured in a TA instrument (Q600) via thermogravimetric analysis: 50 Ncm3·min−1 of air was fed in the chamber and temperature was increased up to 1000 °C.
The plant employed for stability tests is made up of an electrical furnace, where a fluidized bed reactor can be placed. The reactor was filled with 0.6 g of catalyst and 2.4 g of CeO2-SiO2 (acting as filler). Before every test, the loaded sample was reduced for 1 h at 600 °C under a 5%H2/Ar stream. The simulated bioethanol mixture, fed at a rate of 31.6 h−1, can be sent to a boiler for vaporization; in the latter tank, argon is also added as diluent. The latter stream, having a volumetric composition of 10% ethanol, 40% water and 50% argon, reaches the reactor through a traced line; 3.6 Ncm3·min−1 of oxygen are sent to the reactor via an independent line. The weight hourly space velocity (WHSV), referring to the ethanol mass flow rate, was fixed to 60 h−1. The product gas distribution was monitored via a Mass Spectrometer provided by Hiden Analytical. The performance of the catalysts were compared in terms of ethanol conversion (X), hydrogen yield (Y) and carbon formation rate (CFR), based on the results of thermogravimetric analysis of the spent catalysts, which were defined according to Equations (1)–(3), respectively.
3. Results & Discussion
3.1. Characterization of the Fresh Catalysts
Table 1 summarizes the results of characterization in terms of BET surface area and hydrogen consumption recorded during TPR analysis.

Table 1.
Results of fresh catalysts characterization.
Due to the choice of silica as support, all the prepared catalysts displayed surface areas higher than 200 m2·g−1 and only a slight reduction of the final area was recorded upon active species deposition. The employment of materials with high surface area was shown to be a useful tool in order to minimize active phase sintering and improve the activity as well as the stability of the final catalyst [11].
The comparison between the expected and calculated hydrogen uptake revealed that, for all the catalysts investigated in this work, the hydrogen consumption exceeded the theoretical values. These results, enhanced by the addition of the noble metals, can be ascribed to the occurrence of spillover phenomena: active metals such as Ni, Pt, Pd, Ru, Rh have been reported to significantly lower the reduction temperature of cerium oxide. Thus, hydrogen adsorbs onto the metal phase in a dissociative way and the resulting atomic hydrogen can be transported to the oxide support [12]. As a consequence, the calculated uptake was enhanced with respect to the values calculated only accounting for the active metals. This easier hydrogen transfer trough the catalyst was shown to improve the reforming activity [13].
3.2. Stability Evaluation during Oxidative Steam Reforming of Ethanol
The trend of ethanol conversion and hydrogen yield as a function of time-on-stream over the various catalysts is reported in Figure 1.
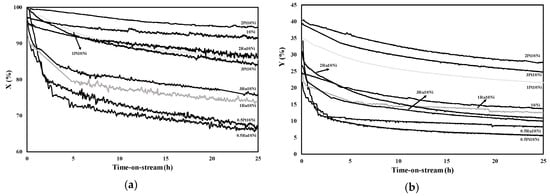
Figure 1.
(a) Ethanol conversion; (b) hydrogen yield over Ni-based catalyst as a function of time-on-stream; P = 1 atm, T = 500 °C; C2H5OH:H2O:O2:Ar = 1:4:0.5:4.5, WHSV = 60 h−1.
Both ethanol conversion and hydrogen yield decreased with time-on-stream, indicating that deactivation phenomena occurred, irrespective of the chosen noble metal and its loading. However, the lowest activity loss was recorded over the 2Pt-10Ni/CeO2-SiO2 catalyst, which displayed a variation in ethanol conversion from 100% to 95% and a reduction in hydrogen yield from 40% to 30% during 25 h of test. The monometallic catalyst reached quite a high conversion at the end of the test, with final values increased with respect to the Pt-Ni as well as Ru-Ni formulations. Conversely, hydrogen yield was reduced in comparison to the 1Pt-Ni, 2Pt-Ni and 3Pt-Ni catalysts, which demonstrated that the noble metal has a crucial role in promoting the pathways involved in H2 production. However, the samples containing 0.5 wt% of Pt or Ru displayed very poor stability. In addition, under the selected operative conditions, ruthenium addition on the Ni/CeO2-SiO2 was not capable of improving the catalyst resistance towards deactivation. From these results, it is possible to conclude that low amounts of noble metals are even detrimental in terms of stability. In addition, for the Pt-based series, the stability performance increased with the Pt loading up to 2 wt%; a further growth in the platinum content did not allow any benefit in terms of resistance towards deactivation.
3.3. Characterization of the Spent Catalysts
The catalysts, after the stability tests described in Figure 1, were characterized to evaluate the effect of time on the stream on their specific surface areas as well as the extent of carbon deposition. The BET area of the spent catalysts and the relative carbon formation rate (defined in Equation (3)) are listed in Table 2.

Table 2.
Results of spent catalysts characterization.
The Ru-based catalysts displayed a more pronounced surface area decrease compared to the Pt-Ni/CeO2-SiO2 sample. In addition, the carbon formation rate grew from 5.1·10−6 of the 1 wt% Ru sample to 7.9·10−6 of the 0.5Ru-Ni gcoke·gcarbon,fed−1·gcatalyst−1·h−1. These results are in line with the marked deactivation observed over the latter catalysts, attested by the quite high rate of coke formation—carbon deposits may occlude the catalyst pores, thus limiting the access of reactants to the catalyst surface [14]. For the most stable sample (2Pt-Ni/CeO2-SiO2), the surface area reduction was only of 15% while CFR was as low as 1.5·10−6 gcoke·gcarbon,fed−1·gcatalyst−1·h−1. Moreover, the Pt-catalyst having a noble metal content of 0.5 wt% displayed the highest carbon formation rate among the tested catalysts, which is in line with the pronounced decrease of ethanol conversion and hydrogen yield observed in Figure 1. The results shown in Table 2 demonstrated that catalyst deactivation was mainly caused by carbon deposition and that the 2Pt-10Ni/CeO2-SiO2 catalyst was less susceptible to deactivation induced by coke.
4. Conclusions
The Pt-Ni and Ru-Ni/CeO2-SiO2 catalysts with various platinum and ruthenium loading (0, 1, 2, 3 wt%) were prepared by sequential wet impregnation and the oxidative steam reforming of ethanol was investigated in terms of ethanol conversion, hydrogen yield and carbon formation rate. The results of stability tests showed that the severe operative condition selected (high space velocity) caused a decrease in the performance of all the catalysts with time-on-stream. However, the catalyst resistance towards deactivation increased with the noble metal up to 2 wt% while, a further growth in Pt as well as Ru content did not assess any benefit. The Pt-based samples were found to be more stable than the Ru-Ni/CeO2-SiO2 catalyst, which underwent a more pronounced surface area decrease and displayed quite high carbon formation rates. After 25 h of test, the 2Pt-10Ni/CeO2-SiO2 catalyst displayed 95% of ethanol conversion and 30% of hydrogen yield. Thus, the latter catalyst was shown to be an effective sample to improve stability and reduce carbon deposition during oxidative steam reforming of ethanol.
Author Contributions
Conceptualization, V.P. and C.R.; methodology, M.C.; formal analysis, V.P.; investigation, C.R. and M.M.; resources, M.C.; data curation, M.M. and M.C.; writing—original draft preparation, C.R. and M.C.; writing—review and editing, V.P. and M.M.; visualization, C.R., M.C. and M.M.; supervision, V.P.; project administration, V.P.; funding acquisition, V.P. All authors have read and agreed to the published version of the manuscript.
Funding
This work has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 734561.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Luo, L.; Zhang, T.; Zhang, X.; Yun, R.; Lin, Y.; Zhang, B.; Xiang, X. Enhanced Hydrogen Production from Ethanol Photoreforming by Site-Specific Deposition of Au on Cu2O/TiO2 pn Junction. Catalysts 2020, 10, 539. [Google Scholar] [CrossRef]
- Łamacz, A.; Jagódka, P.; Stawowy, M.; Matus, K. Dry Reforming of Methane over CNT-Supported CeZrO2, Ni and Ni-CeZrO2 Catalysts. Catalysts 2020, 10, 741. [Google Scholar] [CrossRef]
- Chen, K.; Zhao, Y.; Zhang, W.; Feng, D.; Sun, S. The intrinsic kinetics of methane steam reforming over a nickel-based catalyst in a micro fluidized bed reaction system. Int. J. Hydrogen Energy 2020, 45, 1615–1628. [Google Scholar] [CrossRef]
- Gabriel Rullo, P.; Costa-Castelló, R.; Roda, V.; Feroldi, D. Energy Management Strategy for a Bioethanol Isolated Hybrid System: Simulations and Experiments. Energies 2018, 11, 1362. [Google Scholar] [CrossRef]
- Palma, V.; Ruocco, C.; Cortese, M.; Martino, M. Bioalcohol Reforming: An Overview of the Recent Advances for the Enhancement of Catalyst Stability. Catalysts 2020, 10, 665. [Google Scholar] [CrossRef]
- Mondal, T.; Pant, K.K.; Dalai, A.K. Oxidative and non-oxidative steam reforming of crude bio-ethanol for hydrogen production over Rh promoted Ni/CeO2-ZrO2 catalyst. Appl. Catal. A Gen. 2015, 499, 19–31. [Google Scholar] [CrossRef]
- Palma, V.; Ruocco, C.; Meloni, E.; Gallucci, F.; Ricca, A. Enhancing Pt-Ni/CeO2 performances for ethanol reforming by catalyst supporting on high surface silica. Catal. Today 2018, 307, 175–188. [Google Scholar] [CrossRef]
- Soyal-Baltacıoğlu, F.; Aksoylu, A.E.; Önsan, Z.I. Steam reforming of ethanol over Pt–Ni Catalysts. Catal. Today 2008, 138, 183–186. [Google Scholar] [CrossRef]
- Palma, V.; Ruocco, C.; Meloni, E.; Ricca, A. Renewable Hydrogen from Ethanol Reforming over CeO2-SiO2 Based Catalysts. Catalysts 2017, 7, 226. [Google Scholar] [CrossRef]
- Ruocco, C.; Palma, V.; Ricca, A. Hydrogen production by oxidative reforming of ethanol in a fluidized bed reactor using a PtNi/CeO2SiO2 catalyst. Int. J. Hydrogen Energy 2019, 44, 12661–12670. [Google Scholar] [CrossRef]
- Laosiripojana, N.; Assabumrungrat, S. Catalytic dry reforming of methane over high surface area ceria. Appl. Catal. B Environ. 2005, 60, 107–116. [Google Scholar] [CrossRef]
- Sharma, V.; Crozier, P.A.; Sharma, R.; Adams, J.B. Direct observation of hydrogen spillover in Ni-loaded Pr-doped ceria. Catal. Today 2012, 180, 2–8. [Google Scholar] [CrossRef]
- Fujimoto, K. Spillover of Hydrogen on Carbon and its Role in Catalytic Hydrocarbon Reforming. J. Jpn. Pet. Inst. 1984, 27, 463–471. [Google Scholar] [CrossRef][Green Version]
- Argyle, M.; Bartholomew, C. Heterogeneous Catalyst Deactivation and Regeneration: A Review. Catalysts 2015, 5, 145. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).