Abstract
One-dimensional (1D) nanostructures, such as nanotubes, nanopores, nanodots and nanocones, are characterized by better catalytic properties than bulk materials due to their large active surface area and small geometrical size. There are several methods of synthesis for these structures, including the one- and two-step methods. In the one-step method, a crystal modifier is added to the solution in order to limit the horizontal direction of structures growing during electrodeposition. In this work, cobalt nanoconical structures were obtained from an electrolyte containing CoCl2, H3BO3 and NH4Cl as the crystal modifier. Another method of production of 1D nanocones is electrodeposition of the metal into porous anodic alumina oxide (AAO) templates. This method is called the two-step method. In this case, an AAO template was obtained using two-step anodization. Then, electrodeposition of cobalt was performed from an electrolyte containing CoSO4 and H3BO3. Nanocones obtained by the two-step method show smaller geometrical size. The bulk sample was electrodeposited from the same electrolyte. The electrocatalytic properties of materials fabricated by the one-step and two-step methods were measured in 1M NaOH and compared with bulk materials. Co cones obtained by the one-step method show the worst electrocatalytic properties. The hydrogen evolution reaction started the earliest for Co nanocones electrodeposited in the templates.
1. Introduction
One-dimensional (1D) nanostructures are characterized by two nanometric dimensions in three perpendicular directions. They are characterized by better catalytic properties than bulk materials due to their large active surface area and small geometrical size [1] and can be synthesized using several methods such as electrodeposition with a crystal modifier, two-step anodization and chemical vapor deposition (CVD).
In the case of the one-step method, the addition of a crystal modifier causes the promotion of a parallel direction of structures growth and blocks the horizontal one. This effect is connected with screw dislocation-driven crystal growth [2]. There are several examples of used crystal modifiers such as EDA·2HCl [3], CaCl2·2H2O [2] or NH4Cl [4]. This method allows covering a large area quickly. Fabricated structures have ends with sharp tips. Another advantage of this method is that use of chromic acid is not required, which is dangerous for the environment. In the case of cobalt, shell-like materials are obtained instead of conical ones [5].
The two-step method uses pre-produced templates. They are obtained by two-step anodization, which is a simple and low-cost process. The two-step method allows obtaining round-ended nanocones. The advantage of this method is the possibility of controlling the geometrical features of the synthesized structures. Produced structures show better electrocatalytic properties than bulk materials due to their small geometrical size [6].
Cobalt is an element from the Fe group. It shows ferromagnetic properties. Obtaining conical structures allows applying them as magnetic devices and memory sensors. In this case, they are also characterized by superhydrophobic properties.
In this work, cones were obtained by two methods from the same base electrolyte. They were compared in terms of morphology, real active surface area and electrocatalytic properties.
2. Materials and Methods
The one-step method of the synthesis of conical Co structures consisted of the electrodeposition process. The used electrolyte included the addition of NH4Cl as a crystal modifier. Copper foil acted as a substrate. The electrodeposition process was carried out galvanostatically with a Pt sheet as a counter electrode. The electrodeposition conditions are shown in Table 1.

Table 1.
Conditions of the electrodeposition process in the one-step method.
In the case of the two-step method, the electrodeposition process was conducted in an AAO template. The template was obtained using two-step anodization. This method consisted of a long-period anodization of an aluminum AA1050 sample in 0.3 M oxalic acid at 2 °C for 1 h at 45 V. Then, the obtained Al2O3 layer was removed for 1 h at 60 °C using a mixture of 1.6 wt% chromic acid and 6 wt% phosphoric acid. Alternating short-anodization and pore-widening processes are the second step of anodization. The time of short-anodization was 25 and 20 sec for the first step and next steps, respectively. It was conducted in 0.3 M H2C2O4 at 45 V as well. The temperature of the solution was 9 °C. Pores were winded in 5 wt.% phosphoric acid solution for 12 min at 30 °C. The template with conical pores was obtained after 4 cycles.
The electrodeposition process was conducted using the same condition as in the one-step method for the two-step method and synthesis of the bulk sample. There was no presence of the crystal modifier in the electrolyte. To obtain the free-standing Co nanocones, the template was removed in a dilute NaOH solution. In the case of the bulk material, the copper sample was also the substrate. Surface area for all materials was 2.8 cm2.
Obtained structures were observed using scanning electron microscope JEOL—6000 Plus. SEM photos were also used for determination of the real active surface area.
Electrocatalytic properties of synthesized materials were investigated in the process of hydrogen evolution in a three-electrode cell. The cobalt layer was the working electrode, the platinum sheet was the counter-electrode and the saturated calomel electrode (SCE) was the reference one. The process was carried out at room temperature in 1 M NaOH. The values of EONSET were determined from the figures. EONSET is connected with the start of the hydrogen reaction.
3. Results and Discussion
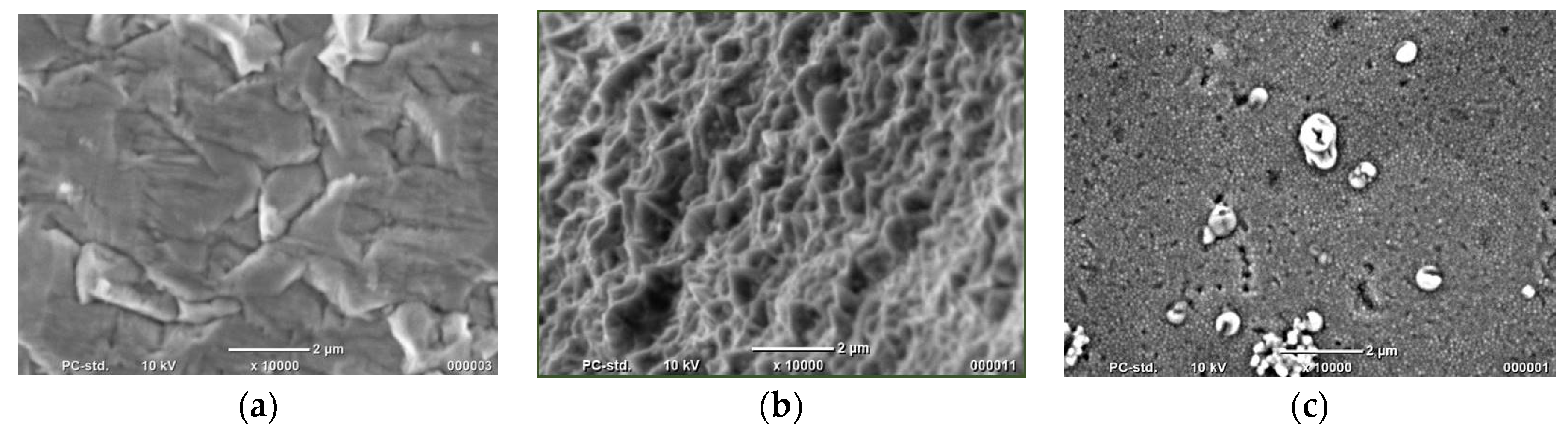
In order to confirm that we obtained the desired structures, samples were observed using SEM JEOL—6000 Plus. The photos are shown in Figure 1.
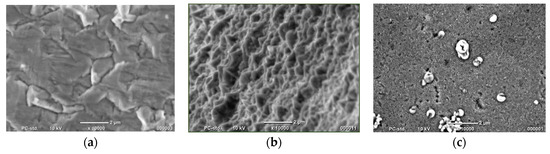
Figure 1.
SEM photos of (a) bulk material and Co nanocones synthesized using (b) the one-step method and (c) two-step method.
Observations confirmed that we obtained all expected types of materials. It can be noticed that the surface of the fabricated bulk material is not a flat one. When synthesized by the one-step method, Co structures are not homogeneous. Single cones are connected, which means that NH4Cl could not block the horizontal direction growth to an appropriate degree. The surface of Co nanocones obtained by the two-step method shows numerous holes and precipitations on the surface. However, their geometrical size is several times smaller.
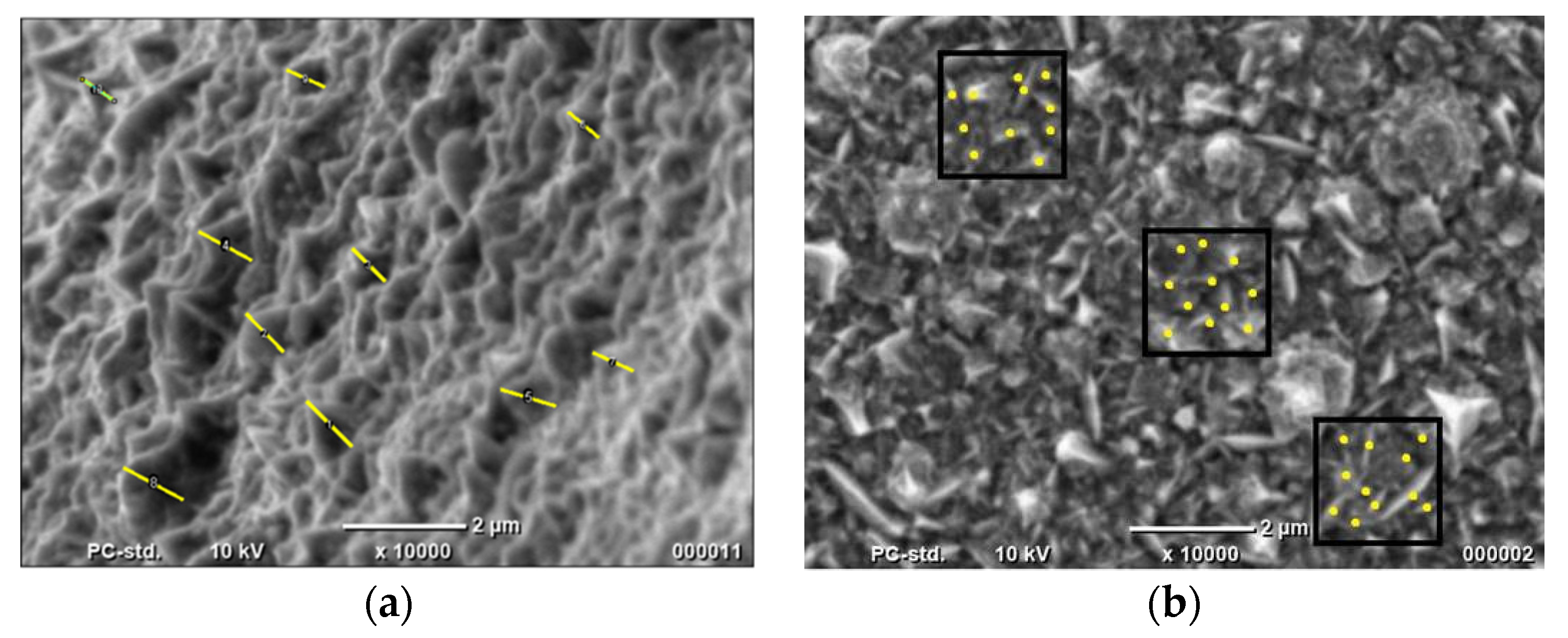
Knowledge about the real surface area is necessary for an appropriate assessment of the electrocatalytic properties of materials. In the case of structures synthesized using the one-step method, the geometrical features were determined using SEM photos. These are shown in Figure 2.
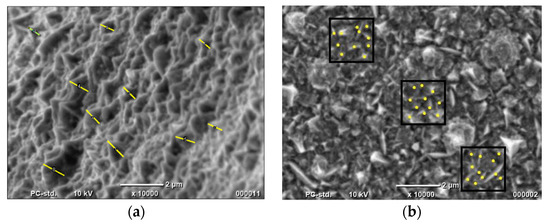
Figure 2.
Determination of (a) nanocones’ height and (b) number of nanocones using SEM photos.
Based on the obtained results, the real surface areas were determined for all materials. Results are shown in Table 2.

Table 2.
Determined values of the real active surface area for all samples.
The results show that the Co nanocones obtained by the one-step method show the greater value of the real active surface area. However, the determination was approximate in all cases. Firstly, the cones are heterogeneous. Their base is also not a round one. Nevertheless, this calculation confirmed that the height of the Co nanocones obtained using the one-step method is almost 10-fold greater than for structures synthesized by the two-step method. The height and the number of conical nanostructures for the two-step method were determined earlier using TEM photos.
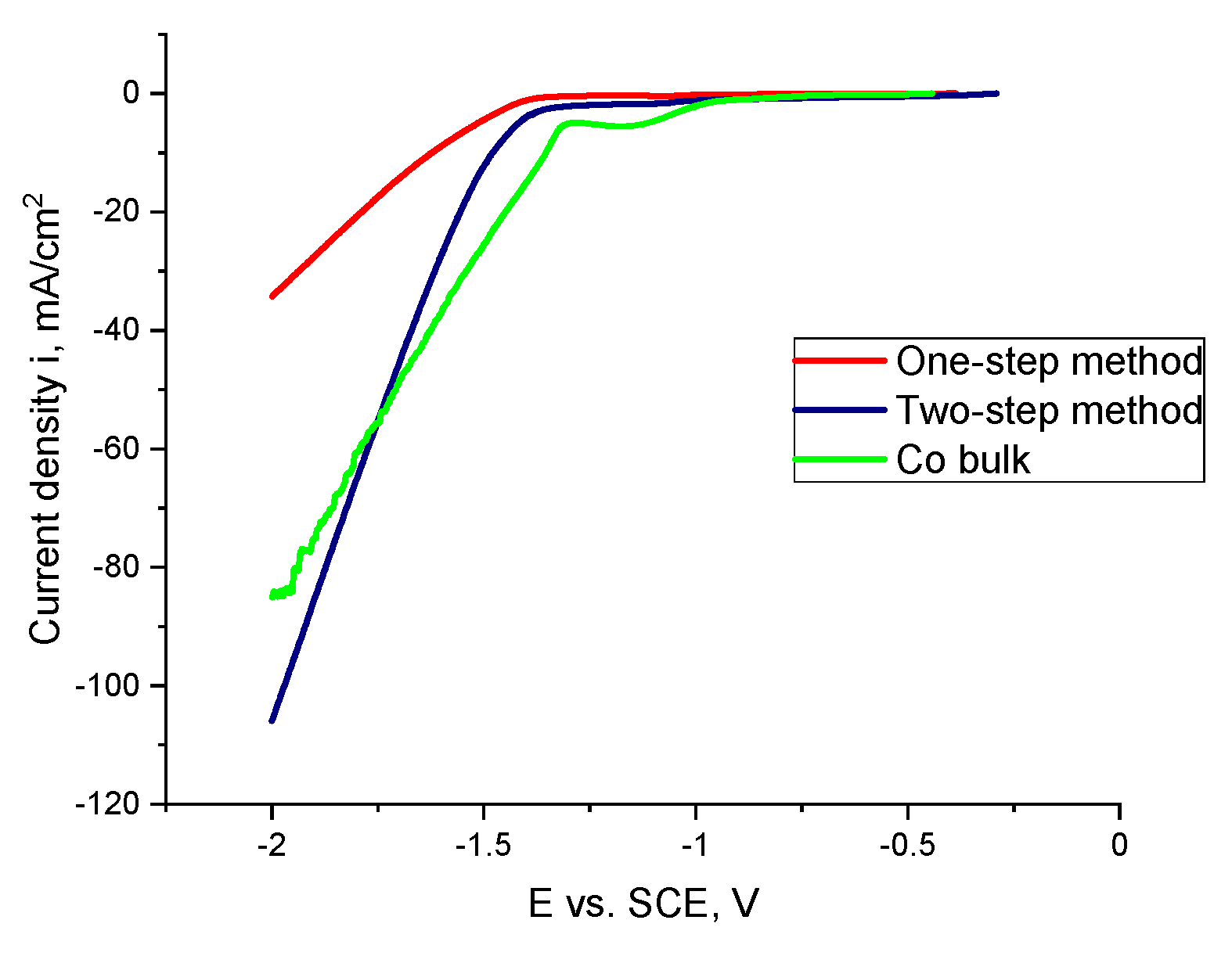
The electrocatalytic properties of samples were measured in 1 M NaOH. The results are shown in Figure 3.
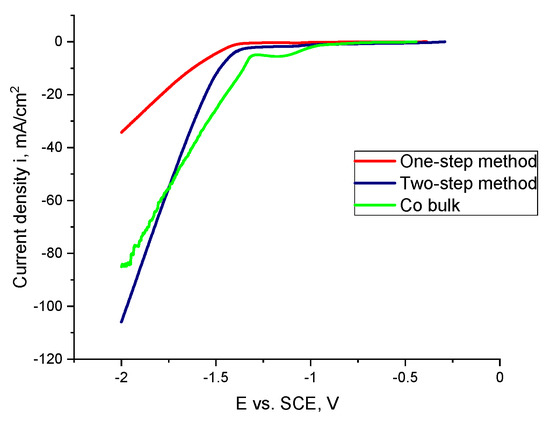
Figure 3.
Linear sweep voltammetry (LSV) curves of Co bulk and Co nanocones obtained using the one-step and two-step method in 1 M NaOH solution.
The obtained results show that the Co structures fabricated by the one-step method show the worst electrocatalytic properties. The sharp characteristic of the curve for the nanoconical Co structures is connected with any of the blocked areas on the sample surface by hydrogen bubbles. The values of the determined EONSET are shown in Table 3.

Table 3.
Values of EONSET for all samples.
It can be noticed that for the Co nanocones obtained by the two-step method, the process began the earliest.
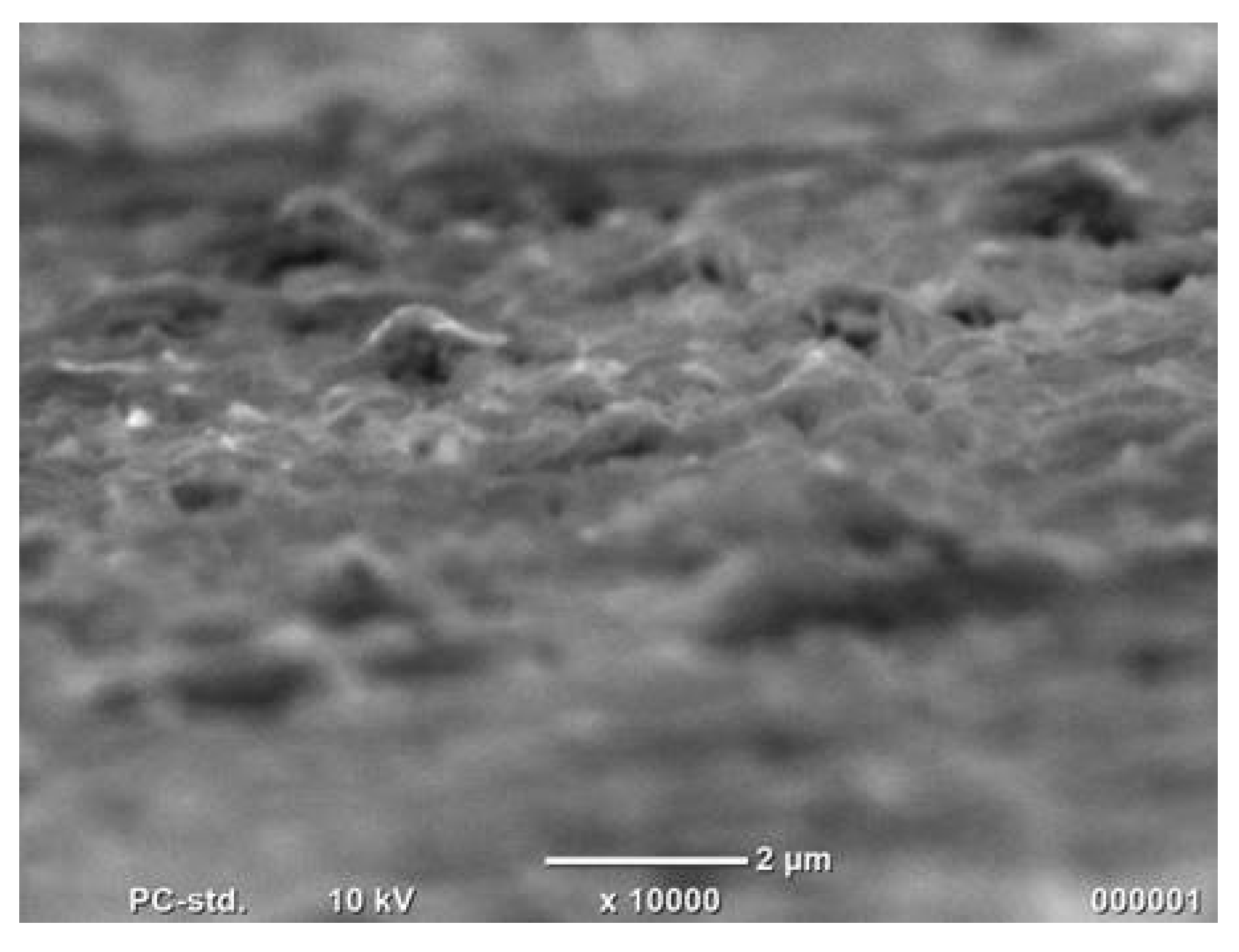
The surface of the Co sample obtained by the one-step method was observed using SEM. It is shown in Figure 4.
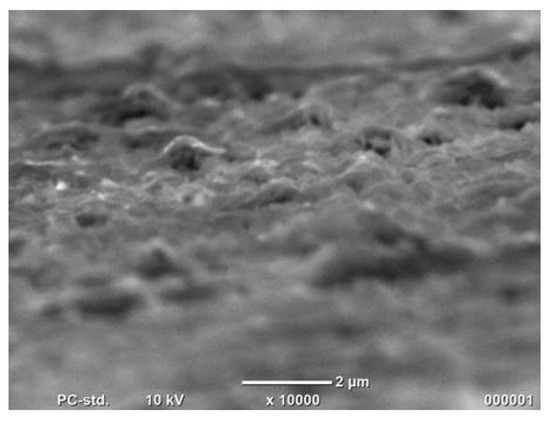
Figure 4.
Co cones obtained using the one-step method after the hydrogen evolution reaction.
There is no noticeable change on the sample surface after the evolution of hydrogen. Unfortunately, the nanoconical Co structures obtained from the template were destroyed during this process.
4. Conclusions
1. It is possible to obtain Co nanocones from electrolytes with the same composition using the one-step and two-step methods.
2. Co structures synthesized by the one-step method are characterized by greater geometrical size. However, there are several microshell structures.
3. The value of the real active surface area was determined approximately using SEM photos. It is connected with heterogeneous Co cones obtained by the one-step method. There was also the assumption that their base is round. Inexactness is connected also with the quality of Co nanocones produced using the two-step method. Their height and diameter were determined earlier using TEM photos.
4. The worst electrocatalytic properties are shown by Co cones obtained by the one-step method. This can be connected with assumptions during the determination of the active surface area. However, in this case, the hydrogen evolution reaction started earlier than for the Co bulk.
5. The hydrogen evolution reaction started the earliest for Co nanocones fabricated by the two-step method.
6. The SEM photo after the hydrogen evolution reaction does not show any change on the sample surface. Taking a photo of the Co nanocones after this reaction was impossible due to the destruction of the layer by the hydrogen bubbles. This can be noticed in the sharp characteristic of the curve.
Author Contributions
K.S., preparation of the AAO templates, electrodeposition of Co by the one-step method, writing—original draft preparation. K.K.-S., SEM analysis. D.K., measurement of electrocatalytic properties. A.J., electrodeposition of Co. P.Z., supervision, writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Polish National Centre of Science, grant number UMO-2016/23/G/ST5/04058.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Kurowska, E.; Brzozka, A.; Jarosz, M.; Sulka, G.D.; Jaskula, M. Silver nanowire array sensor for sensitive and rapid detection of H2O2. Electrochim. Acta 2013, 104, 439–447. [Google Scholar] [CrossRef]
- Lee, J.M.; Jung, K.K.; Lee, S.H.; Ko, J.S. One-step fabrication of nickel nanocones by electrodeposition using CaCl2·2H2O as capping reagent. Appl. Surf. Sci. 2016, 369, 163–169. [Google Scholar] [CrossRef]
- Hang, T.; Li, M.; Fei, Q.; Mao, D. Characterization of nickel nanocones routed by electrodeposition without any template. Nanotechnology 2008, 19, 035201. [Google Scholar] [CrossRef] [PubMed]
- Darband, G.B.; Aliofkhazraei, M.; Dolati, A.; Rouhaghdam, A.S. Electrocrystallization of Ni nanocones from chloride-based bath using crystal modifier by electrochemical methods. J. Alloys Compd. 2020, 818, 152843. [Google Scholar] [CrossRef]
- Xiao, H.; Hu, A.; Hang, T.; Li, M. Electrodeposited nanostructured cobalt film and its dual modulation of both superhydrophobic property and adhesiveness. Appl. Surf. Sci. 2015, 324, 319–323. [Google Scholar] [CrossRef]
- Brzozka, A.; Szeliga, D.; Kurowska-Tabor, E.; Sulka, G.D. Synthesis of copper nanocone array electrodes and its electrocatalytic properties toward hydrogen peroxide reduction. Mater. Lett. 2016, 174, 66–70. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).