In Silico Exploration of a Symmetrical Acridine Derivative’s Anti-Alzheimer Activity: Synthesis, AChE/BuChE Binding, and ADMET Prediction †
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
2.2. Molecular Docking
3. Results and Discussion
3.1. Molecular Docking
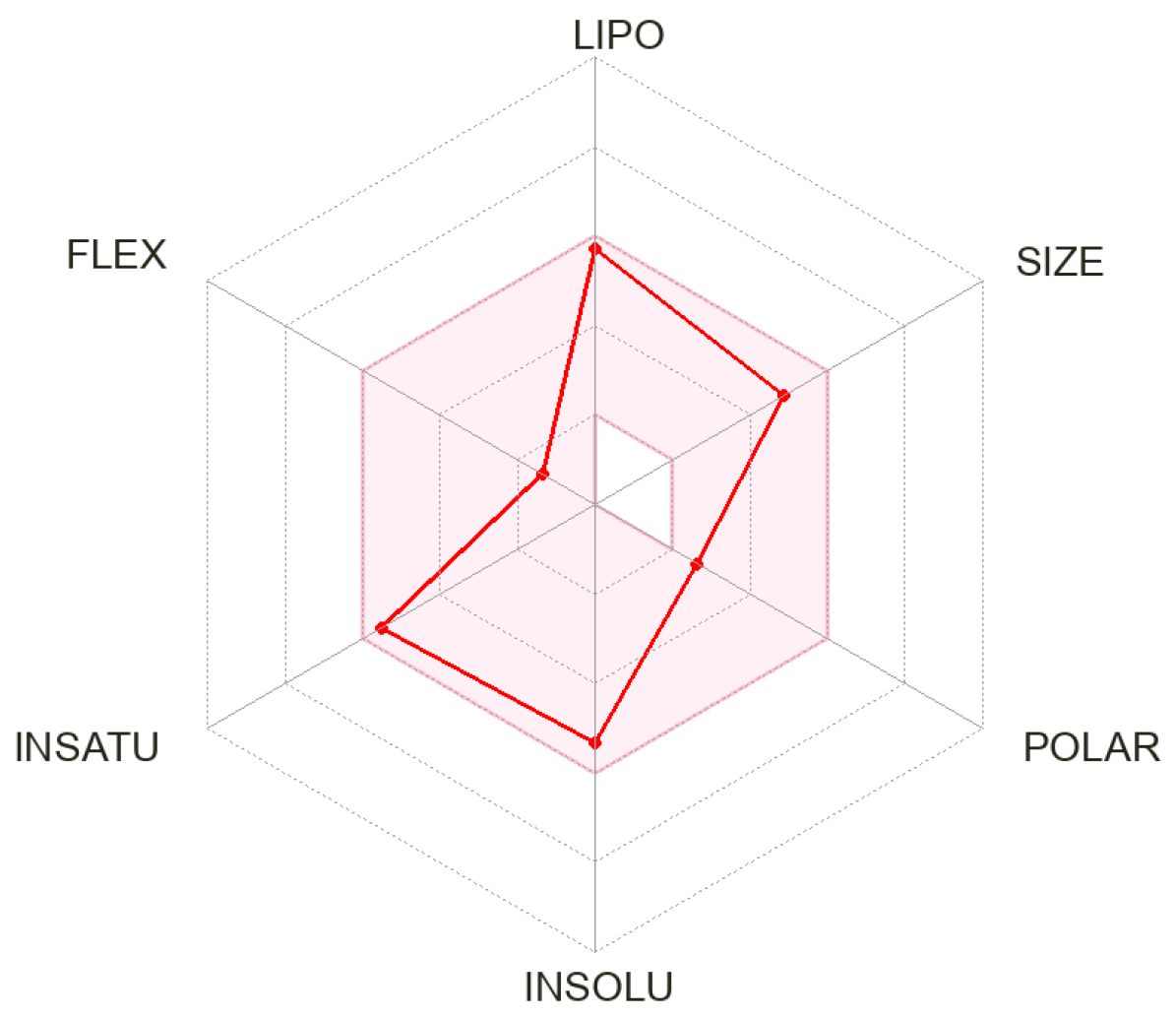
3.2. ADMET Prediction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AChE | Acetylcholinesterase |
| AD | Alzheimer’s disease |
| ADMET | Absorption, distribution, metabolism, excretion, and toxicity |
| BuChE | Butyrylcholinesterase |
| DLS | Drug-likeness score |
| NDs | Neurodegenerative disorders |
References
- Gao, W.; Jing, S.; He, C.; Saberi, H.; Sharma, H.S.; Han, F.; Chen, L. Advancements in neurodegenerative diseases: Pathogenesis and novel neurorestorative interventions. J. Neurorestoratology 2025, 13, 100176. [Google Scholar] [CrossRef]
- Abdallah, A.E. Review on anti-alzheimer drug development: Approaches, challenges and perspectives. RSC Adv. 2024, 14, 11057–11088. [Google Scholar] [CrossRef] [PubMed]
- Grossberg, G.T. Cholinesterase Inhibitors for the Treatment of Alzheimer’s Disease:: Getting On and Staying On. Curr. Ther. Res. 2003, 64, 216–235. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M. Acridine—A neglected antibacterial chromophore. J. Antimicrob. Chemother. 2001, 47, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi-Khanaposhtani, M.; Shabani, M.; Faizi, M.; Aghaei, I.; Jahani, R.; Sharafi, Z.; Shamsaei Zafarghandi, N.; Mahdavi, M.; Akbarzadeh, T.; Emami, S.; et al. Design, synthesis, pharmacological evaluation, and docking study of new acridone-based 1,2,4-oxadiazoles as potential anticonvulsant agents. Eur. J. Med. Chem. 2016, 112, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Sondhi, S.; Bhattacharjee, G.; Jameel, R.; Shukla, R.; Raghubir, R.; Lozach, O.; Meijer, L. Antiinflammatory, analgesic and kinase inhibition activities of some acridine derivatives. Open Chem. 2004, 2, 1–15. [Google Scholar] [CrossRef]
- Makhaeva, G.F.; Lushchekina, S.V.; Boltneva, N.P.; Serebryakova, O.G.; Rudakova, E.V.; Ustyugov, A.A.; Bachurin, S.O.; Shchepochkin, A.V.; Chupakhin, O.N.; Charushin, V.N.; et al. 9-Substituted acridine derivatives as acetylcholinesterase and butyrylcholinesterase inhibitors possessing antioxidant activity for Alzheimer’s disease treatment. Biorg. Med. Chem. 2017, 25, 5981–5994. [Google Scholar] [CrossRef] [PubMed]
- Hamulakova, S.; Imrich, J.; Janovec, L.; Kristian, P.; Danihel, I.; Holas, O.; Pohanka, M.; Böhm, S.; Kozurkova, M.; Kuca, K. Novel tacrine/acridine anticholinesterase inhibitors with piperazine and thiourea linkers. Int. J. Biol. Macromol. 2014, 70, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Piplani, P. Acridine: A Scaffold for the Development of Drugs for Alzheimer’s Disease. Curr. Top. Med. Chem. 2023, 23, 1260–1276. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.-J.; Sun, T.-T.; Yang, Y.-G.; Huang, C.; Chen, X.-B. Divergent synthesis of dual 1,4-dihydropyridines with different substituted patterns from enaminones and aldehydes through domino reactions. RSC Adv. 2018, 8, 12635–12640. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A New Approach for Rapid, Accurate Docking and Scoring. 1. Method and Assessment of Docking Accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of Human Acetylcholinesterase in Complex with Pharmacologically Important Ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef] [PubMed]
- Nachon, F.; Carletti, E.; Ronco, C.; Trovaslet, M.; Nicolet, Y.; Jean, L.; Renard, P.-Y. Crystal structures of human cholinesterases in complex with huprine W and tacrine: Elements of specificity for anti-Alzheimer’s drugs targeting acetyl- and butyryl-cholinesterase. Biochem. J. 2013, 453, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2012, 64, 4–17. [Google Scholar] [CrossRef]


| Entry | Structure | Docking Score (kcal/mol) | |
|---|---|---|---|
| AChE | BuChE | ||
| c |  | −8.704 | −8.620 |
| Galantamine |  | −11.057 | |
| Tacrine |  | −9.178 | |
| Entry | 2D | |
|---|---|---|
| AChE | BuChE | |
| c |  |  |
| Galantamine |  | |
| Tacrine |  | |
| Properties | Compound c |
|---|---|
| Molecular weight (g per mole) | 401.47 |
| Rotatable bonds | 2 |
| H-bond donor | 0 |
| H-bond acceptor | 3 |
| Log Po/w iLOGP | 3.60 |
| Log S ESOL | −5.32 |
| GI | High |
| BBB | Yes |
| Log Kp (cm/s) | −5.57 |
| Bioavailability score | 0.55 |
| TPSA (Å2) | 37.38 |
| DLS score | −0.34 |
| Predicted LD50 (mg/kg) | 1200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouone, Y.O.; Bouzina, A.; Aouf, N.-E. In Silico Exploration of a Symmetrical Acridine Derivative’s Anti-Alzheimer Activity: Synthesis, AChE/BuChE Binding, and ADMET Prediction. Chem. Proc. 2025, 18, 82. https://doi.org/10.3390/ecsoc-29-26743
Bouone YO, Bouzina A, Aouf N-E. In Silico Exploration of a Symmetrical Acridine Derivative’s Anti-Alzheimer Activity: Synthesis, AChE/BuChE Binding, and ADMET Prediction. Chemistry Proceedings. 2025; 18(1):82. https://doi.org/10.3390/ecsoc-29-26743
Chicago/Turabian StyleBouone, Yousra Ouafa, Abdeslem Bouzina, and Nour-Eddine Aouf. 2025. "In Silico Exploration of a Symmetrical Acridine Derivative’s Anti-Alzheimer Activity: Synthesis, AChE/BuChE Binding, and ADMET Prediction" Chemistry Proceedings 18, no. 1: 82. https://doi.org/10.3390/ecsoc-29-26743
APA StyleBouone, Y. O., Bouzina, A., & Aouf, N.-E. (2025). In Silico Exploration of a Symmetrical Acridine Derivative’s Anti-Alzheimer Activity: Synthesis, AChE/BuChE Binding, and ADMET Prediction. Chemistry Proceedings, 18(1), 82. https://doi.org/10.3390/ecsoc-29-26743






