Organocatalytic Synthesis of 2H-Flavenes and Evaluation of Their Reactivity in Michael Versus Knoevenagel Reactions †
Abstract
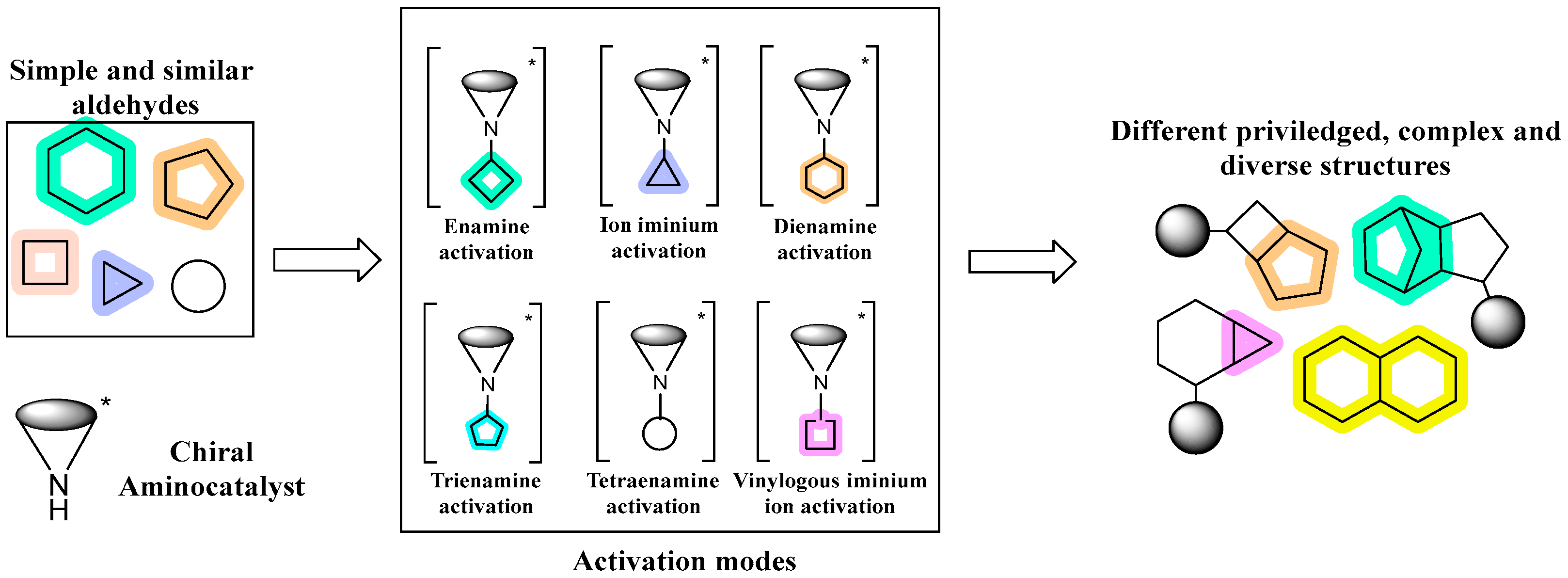
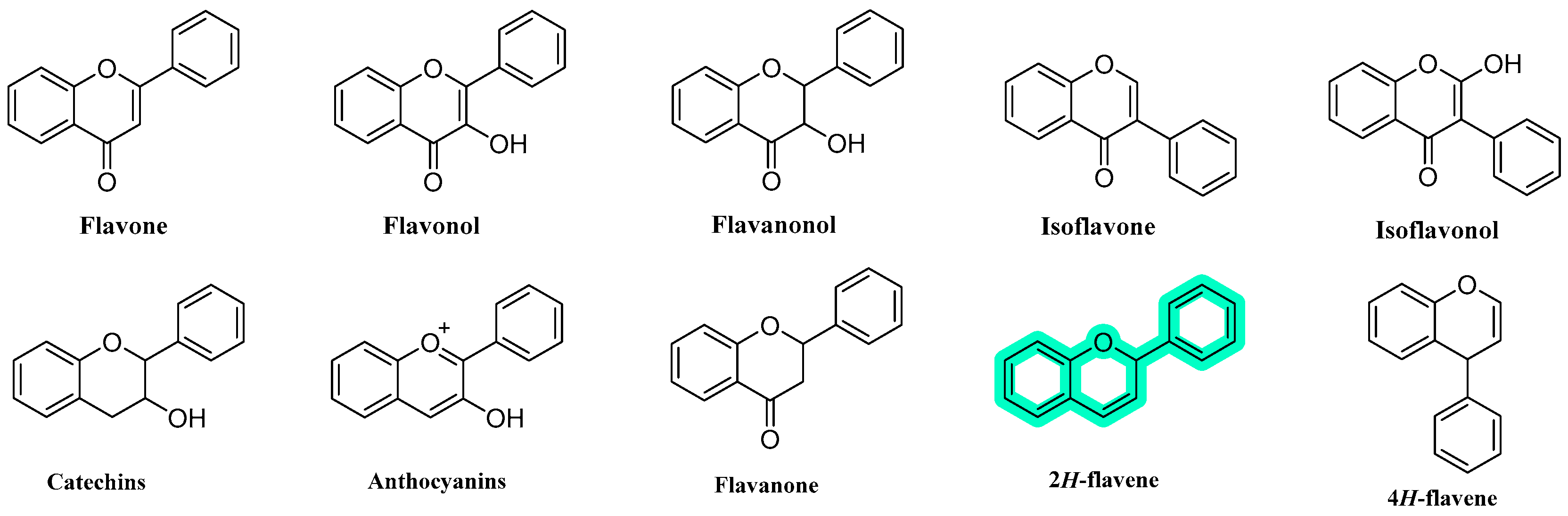
1. Introduction
2. Methodology
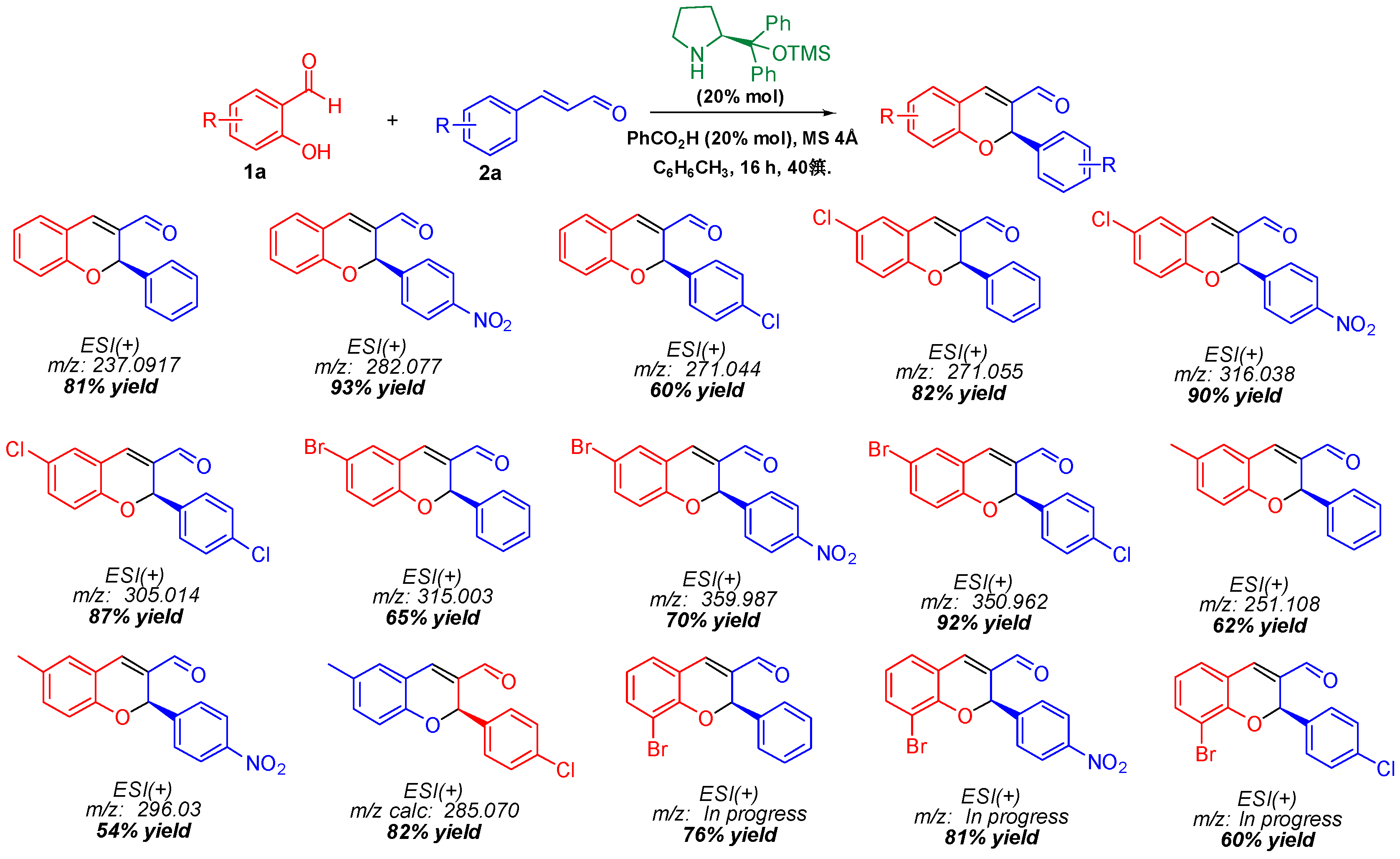
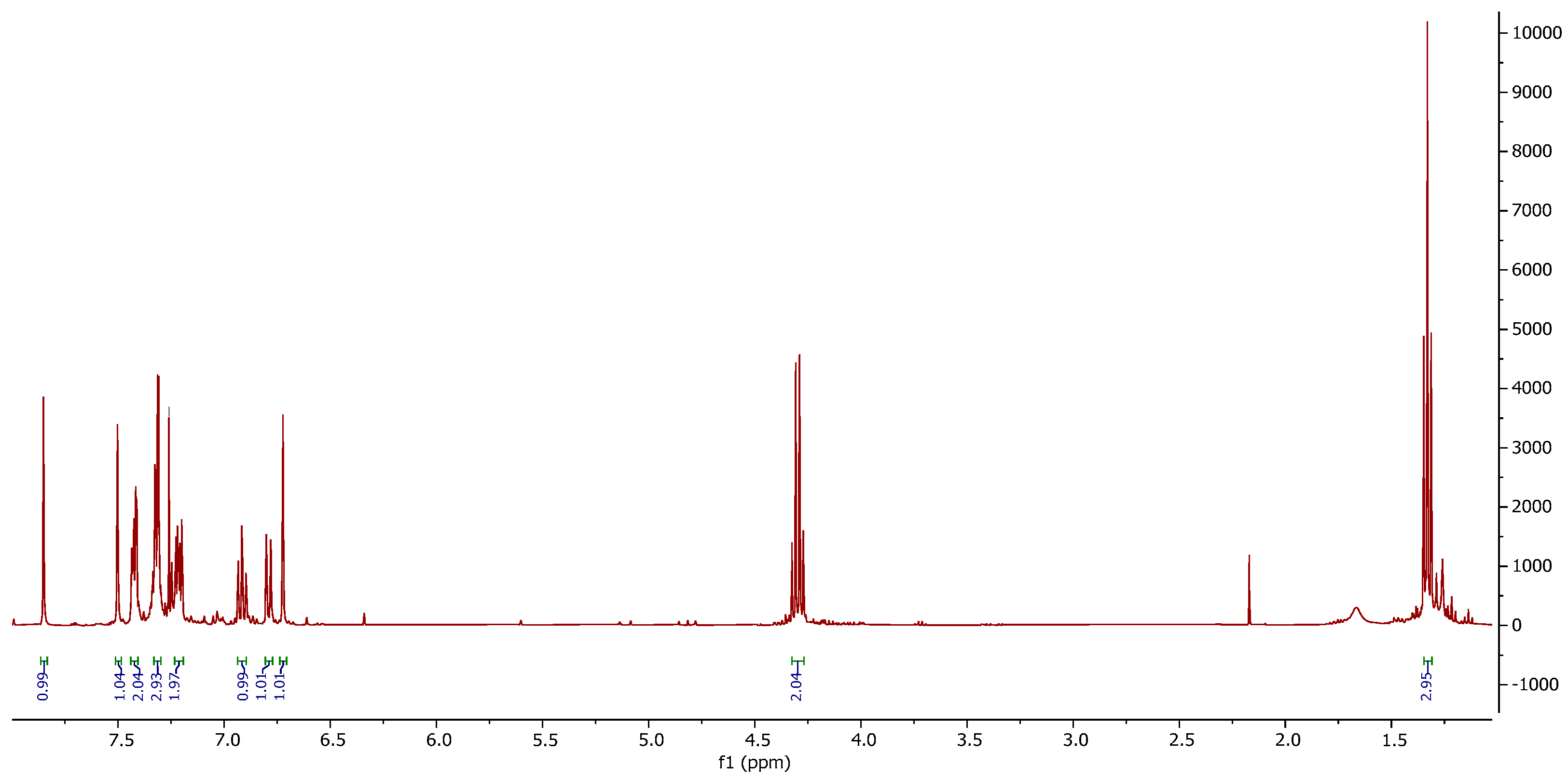
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Welsch, M.E.; Snyder, S.A.; Stockwell, B.R. Privileged scaffolds for library design and drug discovery. Chem. Biol. 2010, 14, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Shereiber, S.L. Target-oriented and diversed-oriented organic synthesis drug discovery. Science 2000, 284, 1964–1969. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Albrecht, L.; Jørgensen, K.A. Aminocatalytic remote functionalization strategies. Chem. Sci. 2013, 4, 2287. [Google Scholar] [CrossRef]
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef]
- Govender, T.; Hojabri, L.; Moghaddam, F.M.; Arvidsson, P.I. Organocatalytic synthesis of chiral benzopyrans. Tetrahedron Asymmetry 2006, 17, 1763. [Google Scholar] [CrossRef]
- Sundén, H.; Ibrahem, I.; Zhao, G.-L.; Eriksson, L.; Córdova, A. Catalytic enantioselective domino oxa Michael/Aldol condensations: Asymmetric synthesis of benzopyran derivatives. Chem. Eur. J. 2007, 13, 574. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; E-Nunu, T.; Zu, L.; Jiang, W.; Wei, S.; Wang, W. One-pot approach to chiral chromenes via enantioselective organocatalytic domino oxa-Michael-aldol reaction. Chem. Commun. 2007, 2007, 507. [Google Scholar] [CrossRef] [PubMed]
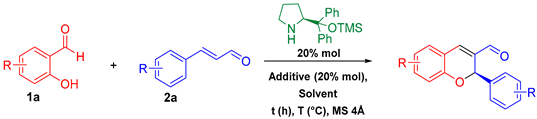 | ||||||||
| Entry | Solvent | T (°C) | Additive | Equiv. 1a | Equiv. 2a | t (h) | Yield (%) | % ee |
| 1 | Toluene | 25 | PhCO2H | 1.2 | 1.0 | 72 | 68 | In progress |
| 2 | Toluene | 25 | p-NO2-PhCO2H | 1.2 | 1.0 | 72 | 60 | In progress |
| 3 | Toluene | 40 | p-NO2-PhCO2H | 1.2 | 1.0 | 16 | 76 | In progress |
| 4 | Toluene | 40 | PhCO2H | 1.2 | 1.0 | 16 | 81 | 90 |
| 5 | Toluene | 40 | PhCO2H | 1.0 | 1.2 | 16 | 51 | 60 |
| 6 | Dioxane | 40 | PhCO2H | 1.2 | 1.0 | 18 | 56 | In progress |
| 7 | Dioxane | 70 | PhCO2H | 1.2 | 1.0 | 16 | 46 | In progress |
| 8 | Chloroform | 40 | PhCO2H | 1.2 | 1.0 | 48 | 44 | In progress |
| 9 | Acetonitrile | 40 | PhCO2H | 1.2 | 1.0 | 48 | 56 | In progress |
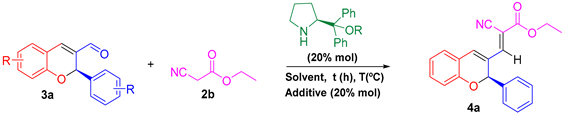 | ||||||
| Entry | Solvent | Temp (°C) | Equiv. 3a | Equiv. 2b | Time (h) | Yield (%) |
| 1 | Chloroform | 25 | 1.0 | 2.0 | 24 | 95 |
| 2 | Toluene | 25 | 1.0 | 2.0 | 24 | 93 |
| 3 | Toluene | 40 | 1.0 | 2.0 | 24 | 86 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mares, X.N.A.; Cruz, D.C.; Gómez, C.V. Organocatalytic Synthesis of 2H-Flavenes and Evaluation of Their Reactivity in Michael Versus Knoevenagel Reactions. Chem. Proc. 2025, 18, 79. https://doi.org/10.3390/ecsoc-29-26699
Mares XNA, Cruz DC, Gómez CV. Organocatalytic Synthesis of 2H-Flavenes and Evaluation of Their Reactivity in Michael Versus Knoevenagel Reactions. Chemistry Proceedings. 2025; 18(1):79. https://doi.org/10.3390/ecsoc-29-26699
Chicago/Turabian StyleMares, Xochitl Netzai Alba, David Cruz Cruz, and Clarisa Villegas Gómez. 2025. "Organocatalytic Synthesis of 2H-Flavenes and Evaluation of Their Reactivity in Michael Versus Knoevenagel Reactions" Chemistry Proceedings 18, no. 1: 79. https://doi.org/10.3390/ecsoc-29-26699
APA StyleMares, X. N. A., Cruz, D. C., & Gómez, C. V. (2025). Organocatalytic Synthesis of 2H-Flavenes and Evaluation of Their Reactivity in Michael Versus Knoevenagel Reactions. Chemistry Proceedings, 18(1), 79. https://doi.org/10.3390/ecsoc-29-26699






