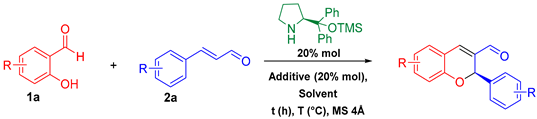
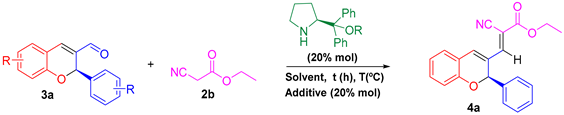
Abstract
In this work we report the stereoselective synthesis of 2H-flavenes via an Aminocatalytic privileged Diversity-Oriented Synthesis (ApDOS) strategy. An oxa-Michael cyclization between salicylaldehydes and an iminium intermediate from cinnamaldehyde and the Hayashi–Jørgensen catalyst produced flavenes at up to 81% yield and 90% ee under optimal conditions (PhCOOH, toluene, 40 °C, 18 h). In general, the reaction proceeds with good yields. Further, reactions with a stabilized carbanion produced Knoevenagel-type adducts, explained by electronic delocalization, HSAB considerations, and kinetic/thermodynamic factors. The resulting polycyclic products show potential as dienophiles in Diels–Alder reactions, offering a valuable framework for future bioactive compound development.
1. Introduction
The stereocontrolled synthesis of privileged structures based on natural architectures constitutes an important topic in contemporary organic chemistry. The ability of such compounds to exert significant biological activity has allowed for the development of a wide variety of synthetic methodologies [1]. Traditionally, the synthesis of bioactive compounds has employed the concept of Target-Oriented Synthesis (TOS), which emphasizes assembling a compound by retrosynthetic analysis. In contrast, Diversity-Oriented Synthesis (DOS) allows for the creation of structurally diverse libraries from common substrates [2].
For years, aminocatalysis has remained an important tool in the field of organocatalysis; its relevance lies in its ability to develop a broad range of methodologies through different catalytic pathways. In this sense, Aminocatalytic privileged Diversity-Oriented Synthesis (ApDOS) has emerged as a versatile strategy that expands structural diversity through various activation modes under stereochemical control (Figure 1) [3].
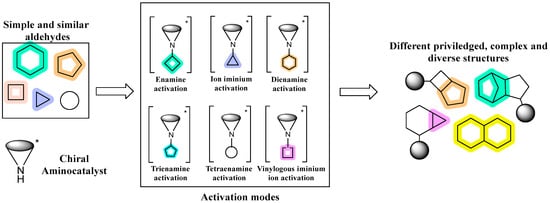
Figure 1.
ApDOS concept.
In terms of privileged frameworks, flavonoids are a large family of secondary metabolites characterized by a C6–C3–C6 structural motif with various pharmacological effects, including antioxidant, antiviral, and anti-inflammatory activities (Figure 2). Particularly, 2H-flavenes are notable for their structural properties and biological potential, which range from the cytotoxic natural product candenatenin E to synthetic derivatives such as acolbifene, a selective estrogen receptor modulator [4].
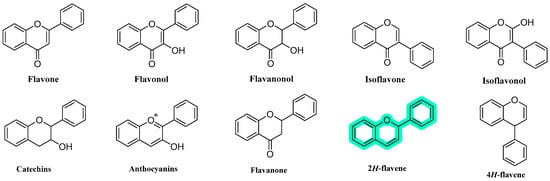
Figure 2.
Classification and structural variants of flavonoid.
Considering the important properties of 2H-flavenes, several methodologies have been stablished for their synthesis, including asymmetric synthesis via aminocatalysis. In 2006, Arvidsson reported the first oxa-Michael route using the Hayashi–Jørgensen catalyst. Later, in 2007, Córdova and Wang improved the selectivity and yields through both the optimization of the conditions and the use of a different organocatalyst [5,6,7].
2. Methodology
All starting materials and reagents employed in this study were commercially sourced unless otherwise specified. Proton 1H NMR spectra were obtained using a Bruker Ascend-400 MHz spectrometer. Flash column chromatography was performed on silica gel using mixtures of hexane/ethyl ether, as well as hexane/ethyl acetate, as eluents.
3. Results and Discussion
Initially, the conditions reported by Córdova and co-workers for flavene synthesis were reproduced. However, optimization was necessary because the initial results were not fully satisfactory (Table 1).

Table 1.
Optimization of the aminocatalytic oxa-Michael reaction for the synthesis of 2H-flavenes.
Once the reaction conditions were optimized (entry 4), the scope and limitations of the reaction were explored by synthesizing derivatives with different substitution patterns on salicylaldehyde and cinnamaldehyde. In this sense, whole products were efficiently obtained through the iminium-ion activation mode, confirming the versatility of the proposed strategy (Scheme 1).
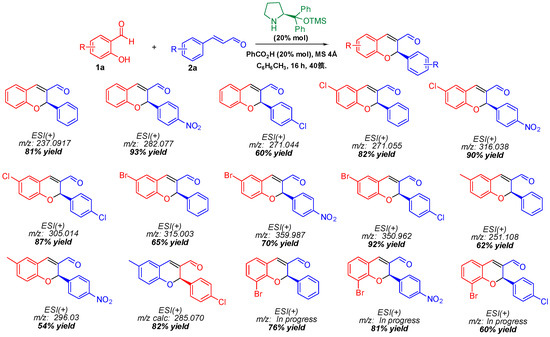
Scheme 1.
Scope and limitations for the synthesis of flavenes.
With the 2H-flavenes in hand, we explored organocatalytic post-functionalization through the Michael addition of a stabilized carbanion to the iminium ion formed by condensation of the aldehyde from the flavene with an aminocatalyst. However, an unexpected Knoevenagel reaction resulted when the enolate from 2b was used (Table 2). This transformation occurred in high yields and was reproducible under different solvents and temperatures.

Table 2.
Knoevenagel functionalization of 2H-flavenes with a stabilized carbanion under different conditions.
This behavior can be explained by considering the electronic delocalization of the 2H-flavene system, as well as Pearson’s Hard–Soft Acid–Base (HSAB) principles and both kinetic and thermodynamic factors, which favor condensation over addition. The formation of the Knoevenagel-type product was confirmed by 1H NMR (Figure 3), high-resolution mass spectrometry (HRMS), and X-ray diffraction.
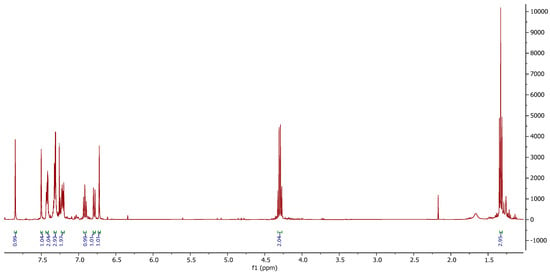
Figure 3.
Representative 1H-NMR signals of the Knoevenagel product.
4. Conclusions
An efficient stereoselective methodology for the synthesis of 2H-flavenes was developed through organocatalysis using iminium-ion activation (up to 81% yield, 90% ee). The strategy is versatile for different salicylaldehydes and cinnamaldehydes. Additionally, post-fuctionalization with a stabilized carbanion was possible for the Knoevenagel-type products rather than Michael adducts, such observed reactivity can be rationalized by electronic delocalization of the flavene system and in accordance with HSAB theory. Overall, this work demonstrates that the ApDOS strategy provides not only enantioselective access to bio-inspired flavenes but also new structural diversifications, providing opportunities for the synthesis of bioactive molecules and new transformations in the future.
Author Contributions
Conceptualization, D.C.C. and C.V.G.; methodology, X.N.A.M.; validation, D.C.C. and C.V.G.; formal analysis, X.N.A.M.; investigation, X.N.A.M.; resources, D.C.C. and C.V.G.; data curation, X.N.A.M.; writing—original draft preparation, X.N.A.M.; writing—review and editing, D.C.C. and C.V.G.; supervision, D.C.C. and C.V.G.; project administration, D.C.C. and C.V.G.; funding acquisition, D.C.C. and C.V.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by SECIHTI, Proyecto de Ciencia Básica y de Frontera 2023-2024 (project code: CBF2023-2024-266).
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
This work was made possible thanks to the support of Becas Na-cionales para estudios de Posgrado 2024-2 (scholarship No. 4024012), and the Laboratorio Nacional de Caracterización de Propiedades Fisicoquímicas y Estructura Molecular (LANCAPFEM) UG.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Welsch, M.E.; Snyder, S.A.; Stockwell, B.R. Privileged scaffolds for library design and drug discovery. Chem. Biol. 2010, 14, 347–361. [Google Scholar] [CrossRef] [PubMed]
- Shereiber, S.L. Target-oriented and diversed-oriented organic synthesis drug discovery. Science 2000, 284, 1964–1969. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Albrecht, L.; Jørgensen, K.A. Aminocatalytic remote functionalization strategies. Chem. Sci. 2013, 4, 2287. [Google Scholar] [CrossRef]
- Liga, S.; Paul, C.; Péter, F. Flavonoids: Overview of Biosynthesis, Biological Activity, and Current Extraction Techniques. Plants 2023, 12, 2732. [Google Scholar] [CrossRef]
- Govender, T.; Hojabri, L.; Moghaddam, F.M.; Arvidsson, P.I. Organocatalytic synthesis of chiral benzopyrans. Tetrahedron Asymmetry 2006, 17, 1763. [Google Scholar] [CrossRef]
- Sundén, H.; Ibrahem, I.; Zhao, G.-L.; Eriksson, L.; Córdova, A. Catalytic enantioselective domino oxa Michael/Aldol condensations: Asymmetric synthesis of benzopyran derivatives. Chem. Eur. J. 2007, 13, 574. [Google Scholar] [CrossRef]
- Li, H.; Wang, J.; E-Nunu, T.; Zu, L.; Jiang, W.; Wei, S.; Wang, W. One-pot approach to chiral chromenes via enantioselective organocatalytic domino oxa-Michael-aldol reaction. Chem. Commun. 2007, 2007, 507. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).