Catalytic Synthesis of Versatile Chiral Heterocycles: En Route to γ-Amino Acid Derivatives †
Abstract
1. Introduction
2. Results and Discussion
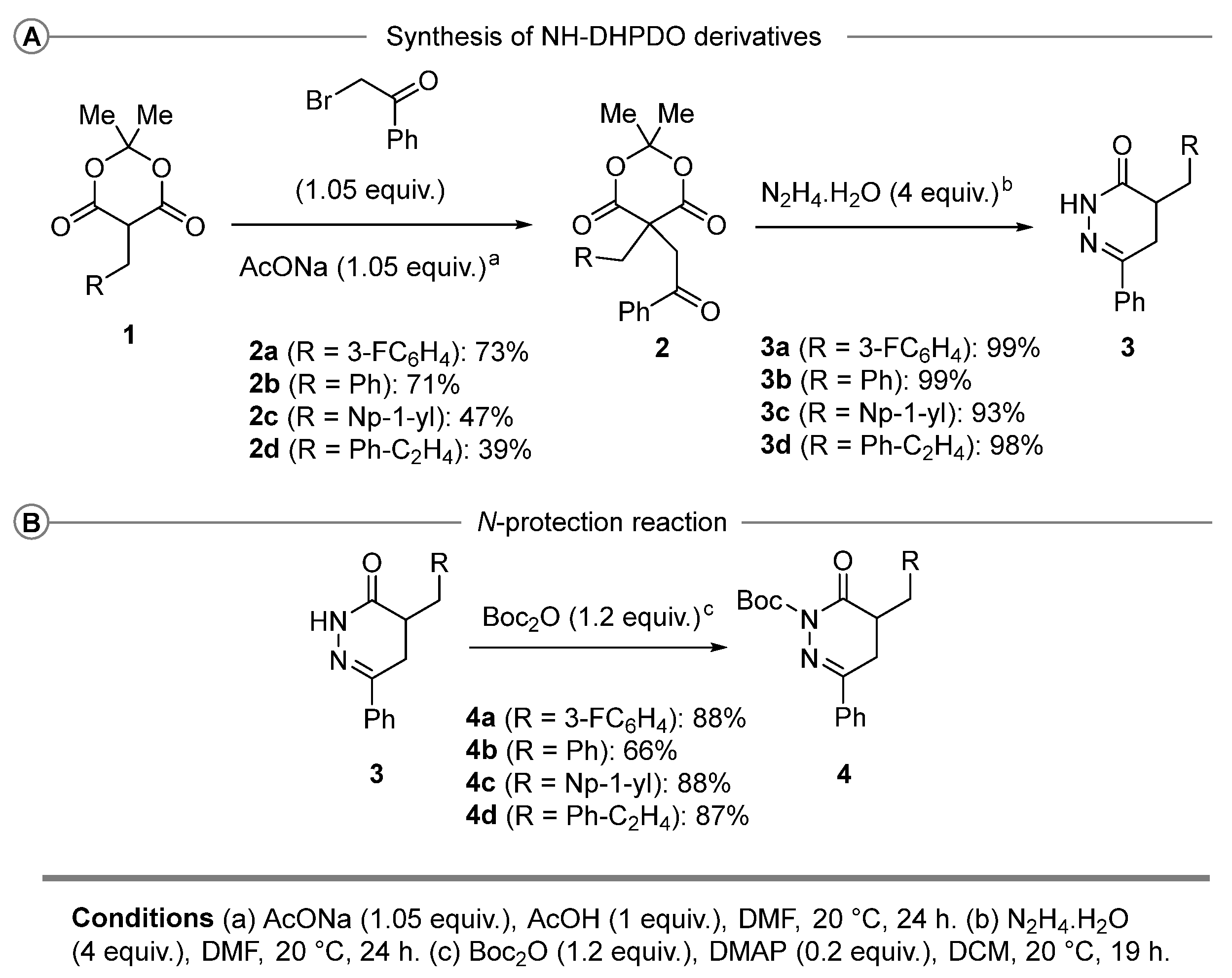
2.1. Synthesis of N-Boc DHPDO Derivatives 4
2.2. α-Functionalization of N-Boc DHPDO Derivatives 4
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ordóñez, M.; Catieviela, C. Stereoselective synthesis of γ-amino acids. Tetrahedron Asymmetry 2007, 18, 3–99. [Google Scholar] [CrossRef]
- Ordóñez, M.; Catieviela, C.; Romero-Estudillo, I. An update on the stereoselective synthesis of γ-amino acids. Tetrahedron Asymmetry 2016, 27, 999–1055. [Google Scholar] [CrossRef]
- Brown, K.M.; Roy, K.K.; Hockerman, G.H.; Doerksen, R.J.; Colby, D.A. Activation of the γ-Aminobutyric Acid Type B (GABAB) Receptor by Agonists and Positive Allosteric Modulators. J. Med. Chem. 2015, 58, 6336–6347. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Halim, H.; Hanrahan, J.R.; Hibbs, D.E.; Johnston, G.A.R.; Chebib, M. A Molecular Basis for Agonist and Antagonist Actions at GABAC Receptors. Chem. Biol. Drug Des. 2008, 71, 306–327. [Google Scholar] [CrossRef] [PubMed]
- Filip, M.; Frankowska, M. GABAB receptors in drug addiction. Pharmacol. Rep. 2008, 60, 755–770. [Google Scholar]
- Marek, I.; Minko, Y.; Pasco, M.; Mejuch, T.; Gilboa, N.; Chechik, H.; Das, J.P. All-Carbon Quaternary Stereogenic Centers in Acyclic Systems through the Creation of Several C–C Bonds per Chemical Step. J. Am. Chem. Soc. 2014, 136, 2682–2694. [Google Scholar] [CrossRef]
- Quasdorf, K.W.; Overman, L.E. Catalytic enantioselective synthesis of quaternary carbon stereocentres. Nature 2014, 516, 181–191. [Google Scholar] [CrossRef]
- Lovering, F. Escape from Flatland 2: Complexity and promiscuity. MedChemComm 2013, 4, 515–519. [Google Scholar] [CrossRef]
- Seebach, D.; Abele, S.; Sifferlen, T.; Hänggi, M.; Gruner, S.; Seiler, P. Preparation and Structure of β-Peptides Consisting of Geminally Disubstituted β2,2- and β3,3-Amino Acids: A Turn Motif for β-Peptides. Helv. Chim. Acta 1998, 81, 2218–2243. [Google Scholar] [CrossRef]
- Mertens, A.; Friebe, W.-G.; Müller-Beckmann, B.; Kampe, W.; Kling, L.; von der Saal, W. Nonsteroidal cardiotonics. 3. New 4,5-dihydro-6-(1H-indol-5-yl)pyridazin-3(2H)-ones and related compounds with positive inotropic activities. J. Med. Chem. 1990, 33, 2870–2875. [Google Scholar] [CrossRef]
- Bansal, R.; Thota, S. Pyridazin-3(2H)-ones: The versatile pharmacophore of medicinal significance. Med. Chem. Res. 2013, 22, 2539–2552. [Google Scholar] [CrossRef]
- Dubey, S.; Bhosle, P.S. Pyridazinone: An important element of pharmacophore possessing broad spectrum of activity. Med. Chem. Res. 2015, 24, 3579–3598. [Google Scholar] [CrossRef]
- Kushwaha, B.; Kushwaha, N.D.; Shaik, B.B.; Chandrasekaran, B.; Obakachi, V.A.; Mokoena, S.; Mohite, S.B.; Karpoormath, R. Pyridazinone: A privileged scaffold for synthetic and biomedical applications. J. Mol. Struct. 2025, 1326, 140948. [Google Scholar] [CrossRef]
- Nieminen, M.S.; Fruhwald, S.; Heunks, L.M.A.; Suominen, P.K.; Gordon, A.C.; Kivikko, M.; Pollesello, P. Levosimendan: Current data, clinical use and future development. Heart Lung Vessels 2013, 5, 227–245. [Google Scholar]
- Ökçelik, B.; Ünlü, S.; Banoglu, E.; Küpeli, E.; Yeşilada, E.; Sahin, M.F. Investigations of New Pyridazinone Derivatives for the Synthesis of Potent Analgesic and Anti-Inflammatory Compounds with Cyclooxygenase Inhibitory Activity. Arch. Pharm. Pharm. Med. Chem. 2003, 336, 406–412. [Google Scholar] [CrossRef]
- Provencher, B.A.; Bartelson, K.J.; Liu, Y.; Foxman, B.M.; Deng, L. Structural Study-Guided Development of Versatile Phase Transfer Catalysts for Asymmetric Conjugate Additions of Cyanide. Angew. Chem. Int. Ed. 2011, 50, 10565–10569. [Google Scholar] [CrossRef]
- Shen, L.-T.; Sun, L.-H.; Ye, S. Highly Enantioselective γ-Amination of α,β-Unsaturated Acyl Chlorides with Azodicarboxylates: Efficient Synthesis of Chiral γ-Amino Acid Derivatives. J. Am. Chem. Soc. 2011, 133, 15894–15897. [Google Scholar] [CrossRef]
- Mao, J.-H.; Wang, Z.-T.; Wang, Z.-Y.; Cheng, Y. N-Heterocyclic Carbene-Catalyzed Oxidative Annulations of α,β-Unsaturated Aldehydes with Hydrazones: Selective Synthesis of Optically Active 4,5-Dihydropyridazin-3-ones and Pyridazin-3-ones. J. Org. Chem. 2015, 80, 6350–6359. [Google Scholar] [CrossRef]
- Zhang, C.-L.; Wang, D.-L.; Chen, K.-Q.; Ye, S. N-Heterocyclic carbene-catalyzed [3 + 3] cyclocondensation of bromoenals with hydrazones: Highly enantioselective synthesis of dihydropyridazones. Org. Biomol. Chem. 2015, 13, 11255–11262. [Google Scholar] [CrossRef]
- Zhang, Z.-J.; Song, J. An isothiourea-catalyzed asymmetric formal [4 + 2] cycloaddition of in situ generated azoalkenes with C1 ammonium enolates. Org. Chem. Front. 2018, 5, 2578–2582. [Google Scholar] [CrossRef]
- Mondal, B.; Maiti, R.; Yang, X.; Xu, J.; Tian, W.; Yan, J.-L.; Li, X.; Chi, Y.R. Carbene-catalyzed enantioselective annulation of dinucleophilic hydrazones and bromoenals for access to aryl-dihydropyridazinones and related drugs. Chem. Sci. 2021, 12, 8778–8783. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Gai, K.; Yuan, Z.; Wu, J.; Lin, A.; Yao, H. Organocatalyzed Formal [4+2] Cycloaddition of in situ Generated Azoalkenes with Arylacetic Acids: An Efficient Approach to the Synthesis of 4,5-Dihydropyridazin-3(2H)-ones. Adv. Synth. Catal. 2015, 357, 3479–3484. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, H.; Xu, J. N-Heterocyclic Carbene-Promoted [4+2] Annulation of α-Chloro Hydrazones with α-Chloro Aliphatic Aldehydes to Access Enantioenriched Dihydropyridazinones. J. Org. Chem. 2022, 87, 3677–3685. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Sharma, D.; Bansal, R. Pyridazinone: An Attractive Lead for Anti-Inflammatory and Analgesic Drug Discovery. Future Med. Chem. 2017, 9, 95–127. [Google Scholar] [CrossRef]
- Kurose, A.; Ishida, Y.; Hirata, G.; Nishikata, T. Direct α-Tertiary Alkylations of Ketones in a Combined Copper–Organocatalyst System. Angew. Chem. Int. Ed. 2021, 60, 10620–10625. [Google Scholar] [CrossRef]
- Wayment, A.X.; Scheidt, K.A. Three-Component Synthesis of γ-Amino Esters with α-Quaternary Carbon Centers via NHC/Photoredox Dual Catalysis. Adv. Synth. Catal. 2025, 367, e70013. [Google Scholar] [CrossRef] [PubMed]
- Ramachary, D.B.; Kishor, M.; Ramakumar, K. A novel and green protocol for two-carbon homologation: A direct amino acid/K2CO3-catalyzed four-component reaction of aldehydes, active methylenes, Hantzsch esters and alkyl halides. Tetrahedron Lett. 2006, 47, 651–656. [Google Scholar] [CrossRef]
- Ramachary, D.B.; Reddy, G.B. Towards organo-click reactions: Development of pharmaceutical ingredients by using direct organocatalytic bio-mimetic reductions. Org. Biomol. Chem. 2006, 4, 4463–4468. [Google Scholar] [CrossRef]
- Tóth, G.; Molnár, S.; Tamás, T.; Borbély, I. An Efficient Synthesis of 4,5-Dihydro-3(2H)-pyridazinone Derivatives. Synth. Commun. 2006, 27, 3513–3523. [Google Scholar] [CrossRef]
- Xu, C.; Qi, Y.; Yang, X.; Li, X.; Li, Z.; Bai, L. Development of C2-Symmetric Chiral Spirocyclic Phase-Transfer Catalysts: Synthesis and Application to Asymmetric Alkylation of Glycinate Schiff Base. Org. Lett. 2021, 23, 2890–2894. [Google Scholar] [CrossRef]
- Xu, C.; Yang, X. Chiral Ammonium Salt Catalyzed Asymmetric Alkylation of Unactivated Amides. Synlett 2022, 33, 664–668. [Google Scholar] [CrossRef]
- Zebrowski, P.; Röser, K.; Chrenko, D.; Pospisil, J.; Waser, M. Enantioselective β-Selective Addition of Isoxazolidin-5-ones to Allenoates Catalyzed by Quaternary Ammonium Salts. Synthesis 2023, 55, 1706–1713. [Google Scholar]


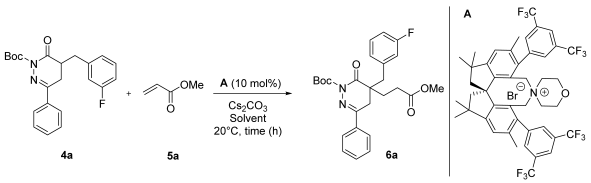
| Entry | Solvent | Cs2CO3 (eq.) | Time (h) | Conv. (%) 2 | 6a (%) 3 | e.r. 4 |
|---|---|---|---|---|---|---|
| 1 | CH2Cl2 | 1.5 | 3 | 90 | 85 | 36:64 |
| 2 | THF | 1.5 | 3 | 95 | 50 | 42:58 |
| 3 | toluene | 1.5 | 3 | 30 | 25 | 10:90 |
| 4 | toluene | 1.5 | 18 | 80 | 75 | 15:85 |
| 5 | toluene | 1.5 | 40 | 100 | 95 | 18:82 |
| 6 | toluene | 3 | 18 | 100 | 95 (54) | 19:81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henry, P.J.; Burel, G.; Nzegge, W.; Waser, M.; Brière, J.-F. Catalytic Synthesis of Versatile Chiral Heterocycles: En Route to γ-Amino Acid Derivatives. Chem. Proc. 2025, 18, 70. https://doi.org/10.3390/ecsoc-29-26715
Henry PJ, Burel G, Nzegge W, Waser M, Brière J-F. Catalytic Synthesis of Versatile Chiral Heterocycles: En Route to γ-Amino Acid Derivatives. Chemistry Proceedings. 2025; 18(1):70. https://doi.org/10.3390/ecsoc-29-26715
Chicago/Turabian StyleHenry, Paul Joël, Gabriel Burel, William Nzegge, Mario Waser, and Jean-François Brière. 2025. "Catalytic Synthesis of Versatile Chiral Heterocycles: En Route to γ-Amino Acid Derivatives" Chemistry Proceedings 18, no. 1: 70. https://doi.org/10.3390/ecsoc-29-26715
APA StyleHenry, P. J., Burel, G., Nzegge, W., Waser, M., & Brière, J.-F. (2025). Catalytic Synthesis of Versatile Chiral Heterocycles: En Route to γ-Amino Acid Derivatives. Chemistry Proceedings, 18(1), 70. https://doi.org/10.3390/ecsoc-29-26715









