Toxicological Assessment of Algerian Honeys: Heavy Metal Contamination as an Indicator of Environmental and Public Health Risks †
Abstract
1. Introduction
2. Materials and Methods
2.1. Honey Samples
2.2. Reagents and Solutions
2.3. Sample Homogenisation and Digestion
2.4. Determination of Mineral Elements
2.5. Trace Metal Determination by ICP-MS
2.6. Data Processing and Statistical Analysis
3. Results and Discussion
3.1. ElementalContent of Algerian Honey Samples
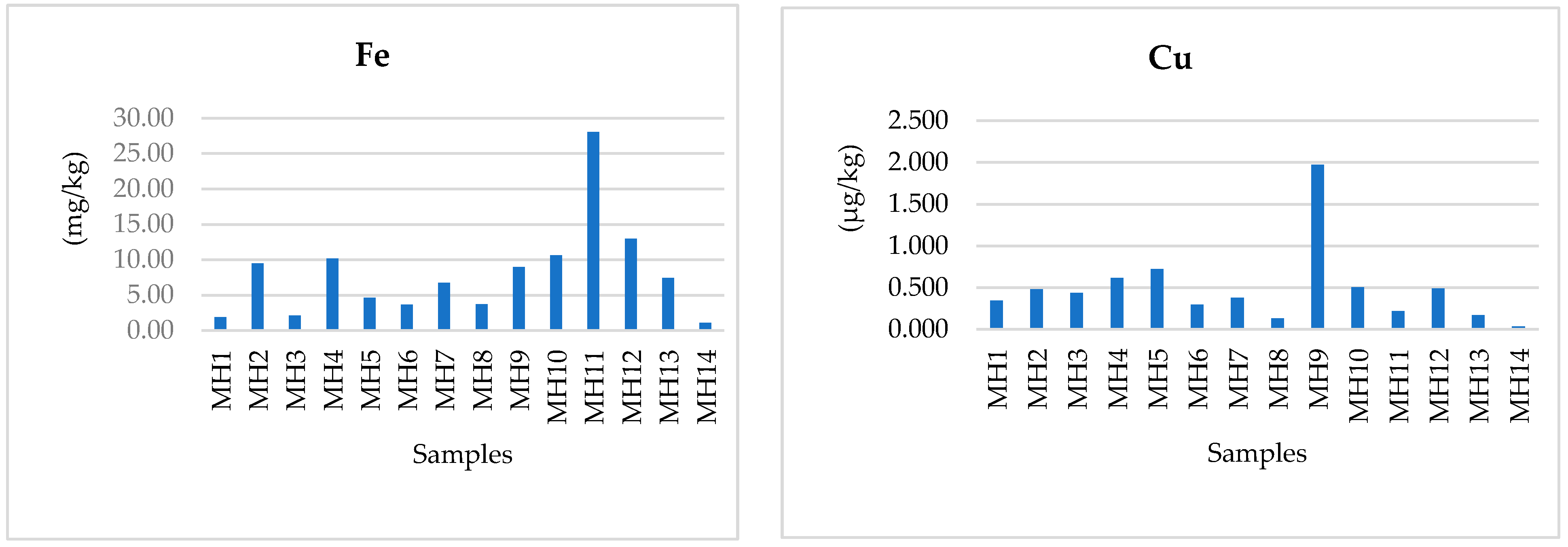
3.2. Essential Micronutrients: Fe, Cu, and Zn
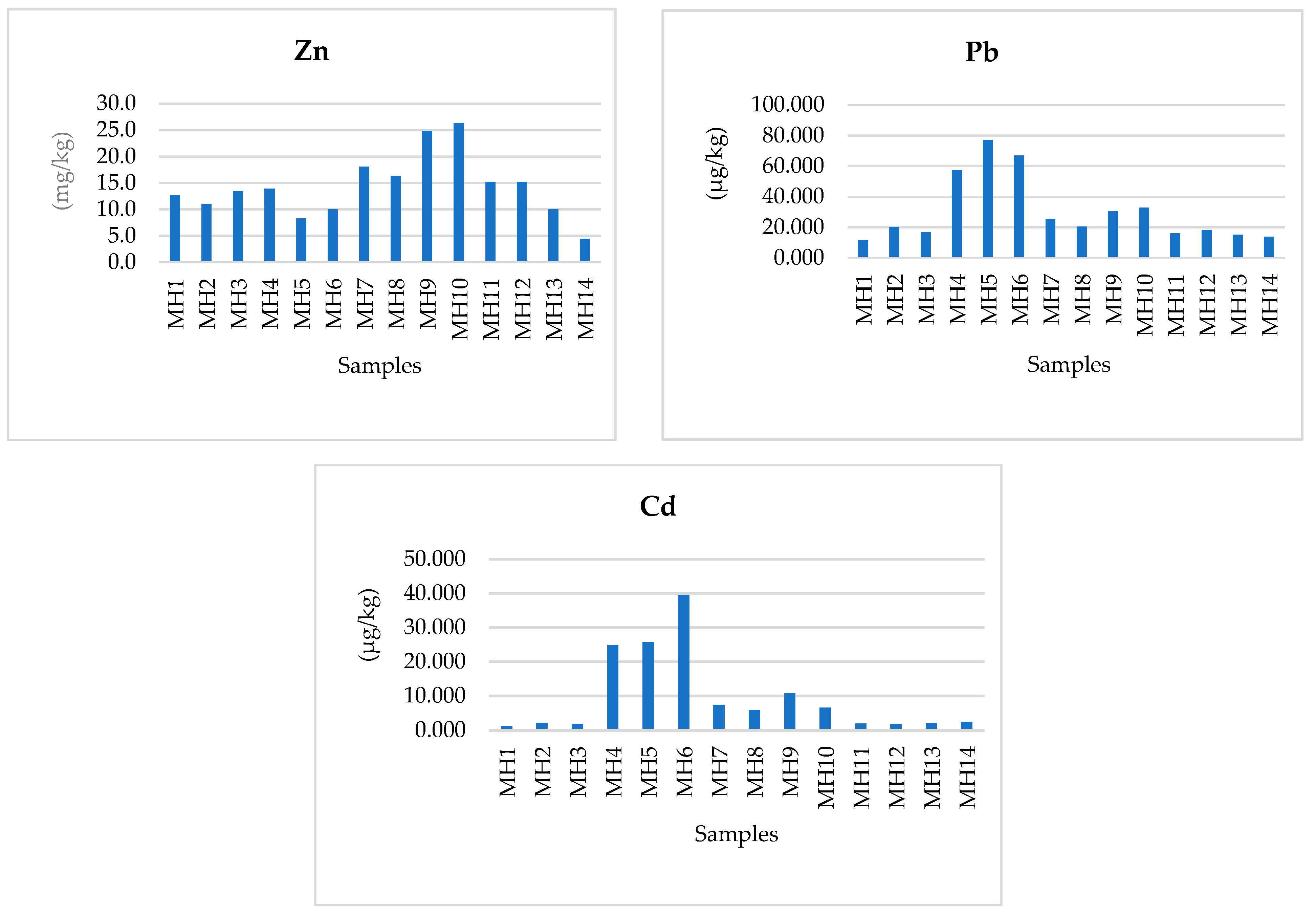
3.3. Toxic Elements: Pb and Cd and Their Health Impacts
| Element | Present Study | Algeria [8] | Morocco [10] N = 29 | Tunisia [11] N = 6 | Portugal [12] N = 16 | France [14] N = 86 | Spain [17] N = 140 | Italy [20] N = 40 | Turkey [6] N = 71 |
|---|---|---|---|---|---|---|---|---|---|
| Fe | 1.11–28.04 | 8.48–59.60 | 1.46–13.95 | 0.83–3.54 | 0.18–2.68 | 0.56–86.76 | 2.26–4.70 | <1–4.4 | <1–7254.62 μg/kg |
| Cu | 0.133–1.975 µg/kg | 1.66–9.62 | ≤0.1 | 0.12–0.34 | 0.00–5.35 | 0.06–1.71 | 0.74–1.88 | 0.06–5.4 | <1–929 μg/kg |
| Zn | 4.40–26.30 | 0.22–13.90 | ≤0.1–0.69 | 0.42–2.06 | 0.03–3.29 | 0.17–6.42 | 2.34–3.47 | <0.5–8.9 | <1–237 μg/kg |
| Pb | 11.515–77.216 µg/kg | 0.54–132.73 μg/kg | ≤0.1 | 0.01–0.05 | - | 0.28–1.08 | 46.32–31.50 μg/kg | 9–209 | <1 μg/kg |
| Cd | 1.119–39.521 µg/kg | 0.24–8.14 μg/kg | - | - | - | 0.08–0.25 | 4.21–4.56 μg/kg | 1.3–4.2 | <1 μg/kg |
3.4. Honey as a Bioindicator of Environmental Quality
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hung, K.-L.J.; Kingston, J.M.; Albrecht, M.; Holway, D.A.; Kohn, J.R. The worldwide importance of honey bees as pollinators in natural habitats. Proc. R. Soc. B 2018, 285, 20172140. [Google Scholar] [CrossRef] [PubMed]
- Burden, C.M.; Morgan, M.O.; Hladun, K.R.; Amdam, G.V.; Trumble, J.J.; Smith, B.H. Acute sublethal exposure to toxic heavy metals alters honey bee (Apis mellifera) feeding behavior. Sci. Rep. 2019, 9, 4253. [Google Scholar] [CrossRef] [PubMed]
- Vareda, J.P.; Valente, A.J.M.; Duraes, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manag. 2019, 246, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Engwa, G.A.; Ferdinand, P.U.; Nwalo, F.N.; Unachukwu, M.N. Mechanism and Health Effects of Heavy Metal Toxicity in Humans. In Poisoning in the Modern World—New Tricks for an Old Dog? Karcioglu, O., Arslan, B., Eds.; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Forte, G.; Fadda, C.; Bocca, B.; Erre, G.L.; Passiu, G.; Madeddu, R. Association between exposure to heavy metals and systemic sclerosis: The levels of Al, Cd, Hg, and Pb in blood and urine of patients. Biol. Trace Elem. Res. 2019, 190, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kılıç Altun, S.; Dinç, H.; Paksoy, N.; Temamoğulları, F.K.; Savrunlu, M. Analyses of mineral content and heavy metal of honey samples from South and East region of Turkey by using ICP-MS. Int. J. Anal. Chem. 2017, 2017, 6391454. [Google Scholar] [CrossRef] [PubMed]
- Kadri, S.M.; Zaluski, R.; Orsi, R.O. Nutritional and mineral contents of honey extracted by centrifugation and pressed processes. Food Chem. 2017, 218, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Bereksi-Reguig, D.; Bouchentouf, S.; Allali, H.; Adamczuk, A.; Kowalska, G.; Kowalski, R. Trace elements and heavy metal contents in west Algerian natural honey. J. Anal. Methods Chem. 2022, 2022, 7890856. [Google Scholar] [CrossRef] [PubMed]
- Bogdanov, S.; Haldimann, M.; Luginbühl, W.; Gallmann, P. Minerals in honey: Environmental, geographical and botanical aspects. J. Apic. Res. 2007, 46, 269–275. [Google Scholar] [CrossRef]
- Moujanni, A.; Partida, L.; Essamadi, A.K.; Hernanz, D.; Heredia, F.J.; Terrab, A. Physicochemical characterization of unique unifloral honey: Euphorbia resinifera. CyTA—J. Food 2017, 16, 27–35. [Google Scholar] [CrossRef]
- Boussaid, A.; Chouaibi, M.; Rezig, L.; Hellal, R.; Donsì, F.; Ferrari, G.; Hamdi, S. Physicochemical and bioactive properties of six honey samples from various floral origins from Tunisia. Arab. J. Chem. 2018, 11, 265–274. [Google Scholar] [CrossRef]
- Silva, L.R.; Sousa, A.; Taveira, M. Characterization of Portuguese honey from Castelo Branco region according to their pollen spectrum, physicochemical characteristics and mineral contents. J. Food Sci. Technol. 2017, 54, 2551–2561. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives. Summary of Evaluations. 1983; TRS 696-JECFA 27/29, FAS 18-JECFA 27/203. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=2859 (accessed on 12 September 2025).
- Devillers, J.; Doré, J.C.; Marenco, M.; Poirier-Duchêne, F.; Galand, N.; Viel, C. Chemometrical analysis of 18 metallic and nonmetallic elements found in honeys sold in France. J. Agric. Food Chem. 2002, 50, 5998–6007. [Google Scholar] [CrossRef] [PubMed]
- Joint FAO/WHO Expert Committee on Food Additives. Summary of Evaluations. 1982; TRS 683-JECFA 26/31, FAS 17-JECFA 26/265. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=2824 (accessed on 12 September 2025).
- Gekière, A.; Vanderplanck, M.; Michez, D. Trace metals with heavy consequences on bees: A comprehensive review. Sci. Total Environ. 2023, 859, 165084. [Google Scholar] [CrossRef] [PubMed]
- Frías, I.; Rubio, C.; González-Iglesias, T.; Gutiérrez, Á.J.; González-Weller, D.; Hardisson, A. Metals in fresh honeys from Tenerife Island, Spain. Bull. Environ. Contam. Toxicol. 2008, 80, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Cao, X.; Hu, Y.; Cheng, H. Source Apportionment and Risk Assessment of Heavy Metals in Agricultural Soils in a Typical Mining and Smelting Industrial Area. Sustainability 2024, 16, 1673. [Google Scholar] [CrossRef]
- Jaishankar, M.; Tseten, T.; Anbalagan, N.; Mathew, B.B.; Beeregowda, K.N. Toxicity, mechanism and health effects of some heavy metals. Interdiscip. Toxicol. 2014, 7, 60–72. [Google Scholar] [CrossRef] [PubMed]
- Conti, M.E.; Canepari, S.; Finoia, M.G.; Mele, G.; Astolfi, M.L. Characterization of Italian multiforal honeys on the basis of their mineral content and some typical quality parameters. J. Food Compost. Anal. 2018, 74, 102–113. [Google Scholar] [CrossRef]
- Joint FAO/WHO Expert Committee on Food Additives. Summary of Evaluations. 1999; TRS 896-JECFA 53/81, FAS 44-JECFA 53/273. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/chemical.aspx?chemID=3511 (accessed on 12 September 2025).
- International Agency for Research on Cancer. Arsenic, Metals, Fibres, and Dusts; International Agency for Research of Cancer: Lyon, France, 2012; Volume 100C. [Google Scholar]
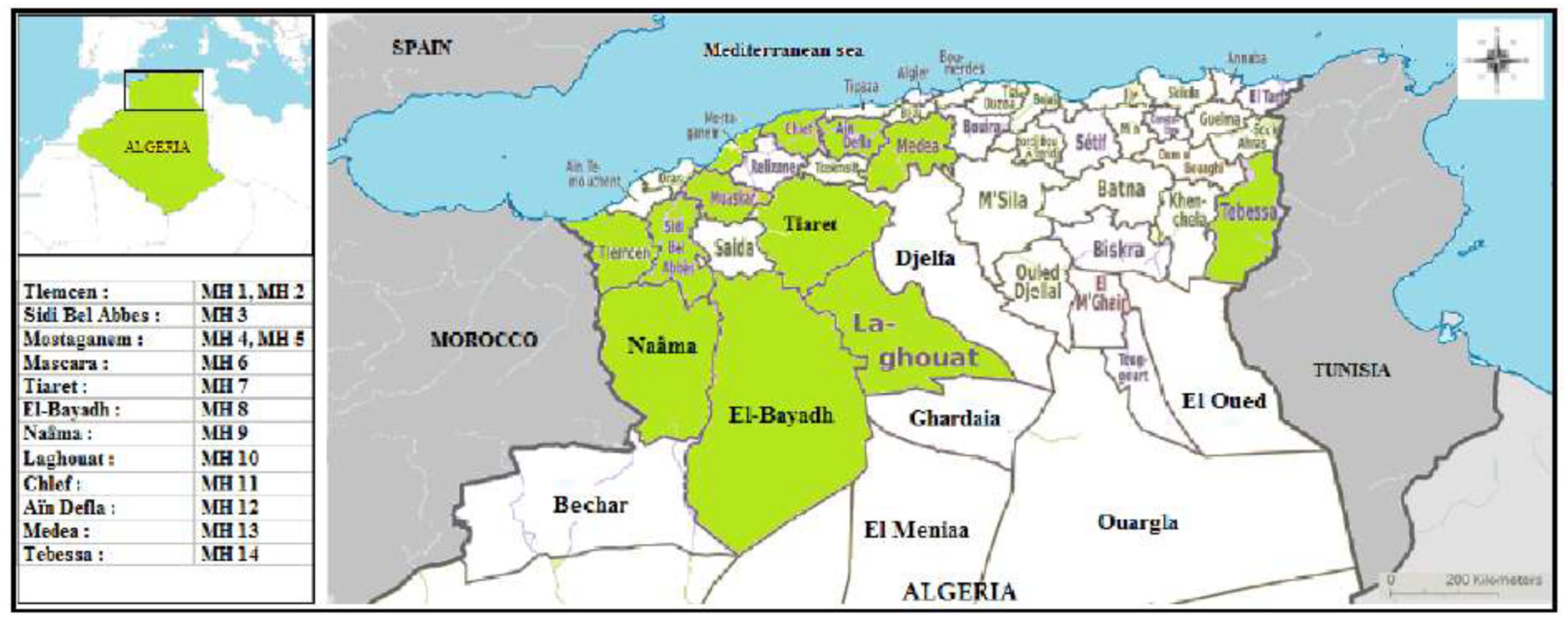
| Region | Sample | Scientific Name | Season/Year of Harvest |
|---|---|---|---|
| Tlemcen | MH 1 | Nasturtium officinale R.Br. | Spring 2022 |
| MH 2 | Thymus ciliatus subsp coloratus | Spring 2022 | |
| Sidi Bel Abbes | MH 3 | Scolymus hispanicus L. | Spring 2022 |
| Mostaganem | MH 4 | Eucalyptus globulus Labill. | Summer 2022 |
| MH 5 | Citrus sinensis L. | Spring 2022 | |
| Mascara | MH 6 | Citrus sinensis L. | Spring 2022 |
| Tiaret | MH 7 | Eucalyptus globulus Labill. | Summer 2022 |
| El-Bayadh | MH 8 | Euphorbia guyoniana Boiss. & Reut. | Summer 2022 |
| Naâma (Mechria) | MH 9 | Ziziphus lotus L. | Summer 2022 |
| Laghouat (Aflou) | MH 10 | Euphorbia guyoniana Boiss. & Reut. | Spring 2022 |
| Chlef | MH 11 | Petroselinum crispum (Mill.) Fuss. | Spring 2022 |
| Aïn Defla | MH 12 | Eucalyptus globulus Labill. | Summer 2022 |
| Medea | MH 13 | Daucus carota L. | Spring 2022 |
| Tebessa | MH 14 | Rosmarinus officinalis L. | Spring 2022 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kazi-Tani, N.; Allali, H.; Puścion-Jakubik, A.; Aissaoui, N.; Socha, K. Toxicological Assessment of Algerian Honeys: Heavy Metal Contamination as an Indicator of Environmental and Public Health Risks. Chem. Proc. 2025, 18, 68. https://doi.org/10.3390/ecsoc-29-26688
Kazi-Tani N, Allali H, Puścion-Jakubik A, Aissaoui N, Socha K. Toxicological Assessment of Algerian Honeys: Heavy Metal Contamination as an Indicator of Environmental and Public Health Risks. Chemistry Proceedings. 2025; 18(1):68. https://doi.org/10.3390/ecsoc-29-26688
Chicago/Turabian StyleKazi-Tani, Nessrine, Hocine Allali, Anna Puścion-Jakubik, Nadia Aissaoui, and Katarzyna Socha. 2025. "Toxicological Assessment of Algerian Honeys: Heavy Metal Contamination as an Indicator of Environmental and Public Health Risks" Chemistry Proceedings 18, no. 1: 68. https://doi.org/10.3390/ecsoc-29-26688
APA StyleKazi-Tani, N., Allali, H., Puścion-Jakubik, A., Aissaoui, N., & Socha, K. (2025). Toxicological Assessment of Algerian Honeys: Heavy Metal Contamination as an Indicator of Environmental and Public Health Risks. Chemistry Proceedings, 18(1), 68. https://doi.org/10.3390/ecsoc-29-26688








