Synthesis and Biological Activity Evaluation In Silico of Bis(4-Hydroxy-6H-1,3-Oxazin-6-One) Derivatives and the Products of Their Alcoholysis †
Abstract
1. Introduction
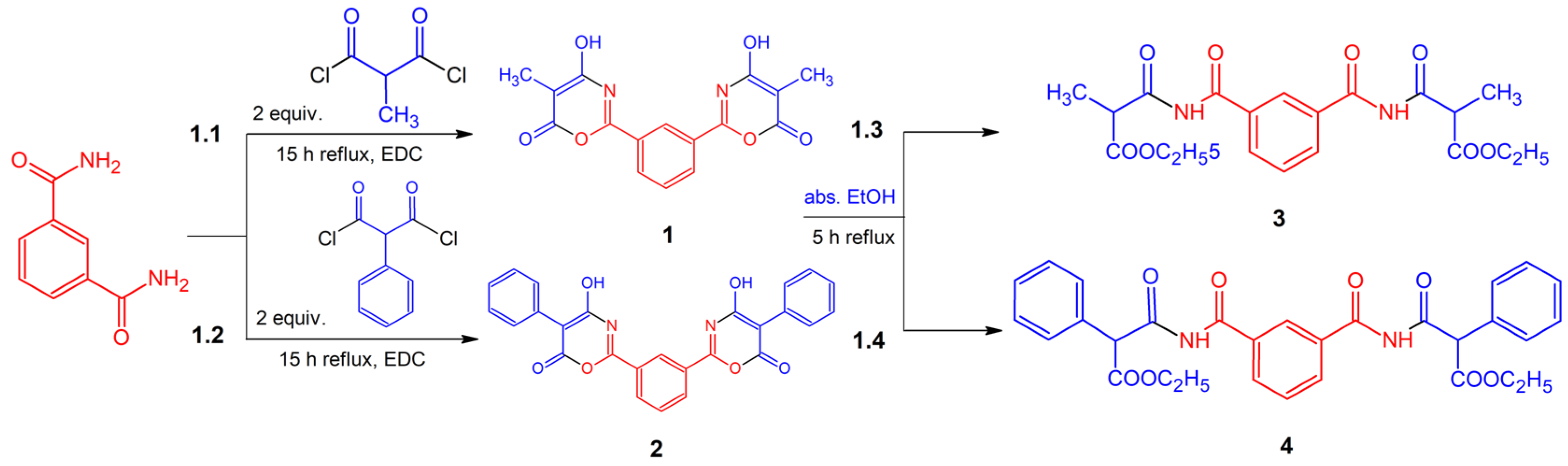
- To carry out the reaction of benzene-1,3-dicarboxamide with a twofold excess of substituted malonyl chloride in 1,2-dichloroethane;
- To implement the interaction of the obtained bis(4-hydroxy-6H-1,3-oxazin-6-ones) with ethanol and to determine the effect of substituents at the C5 position of the oxazine cycle on the yield of the products;
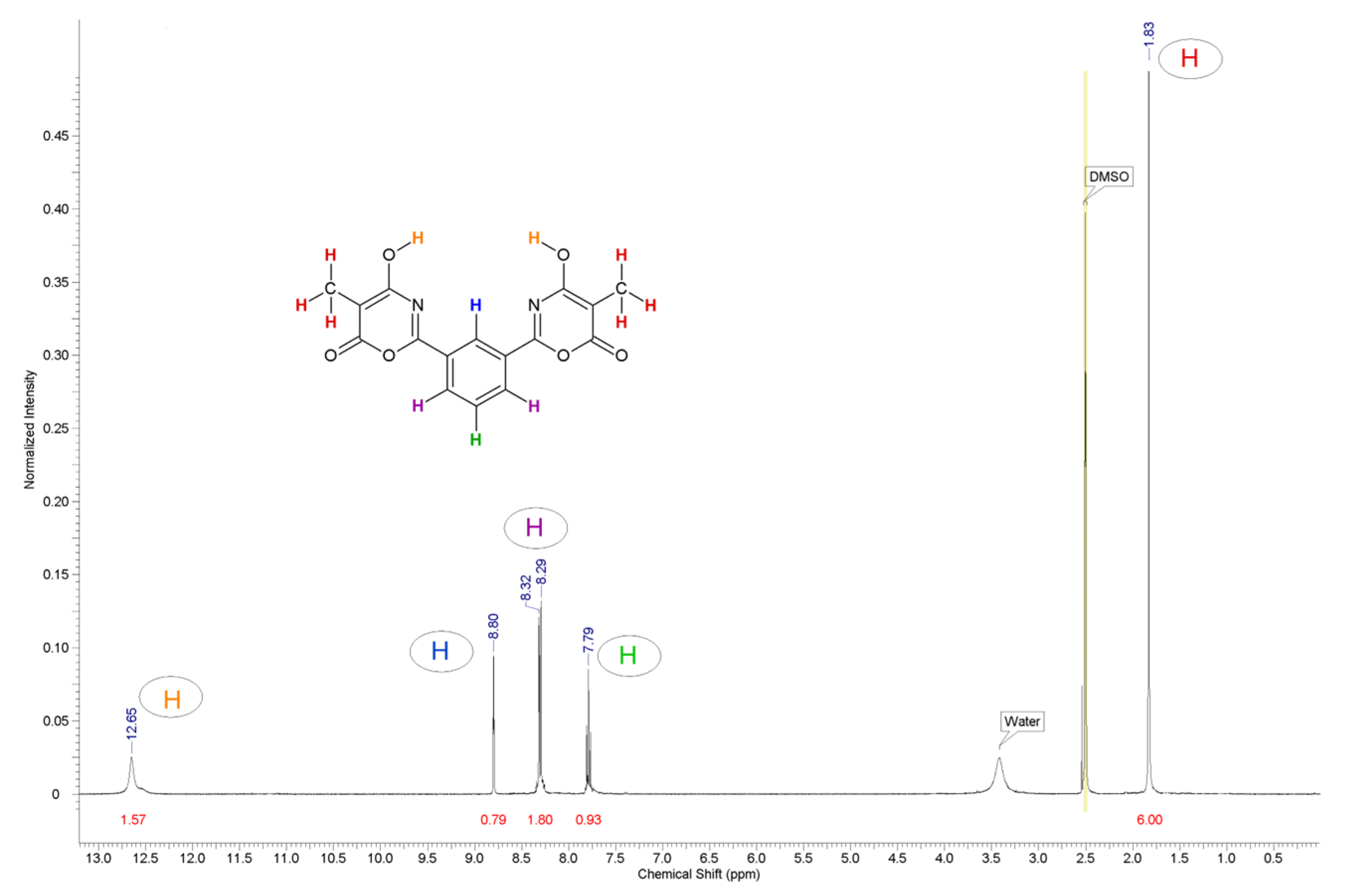
- To prove the structure of the synthesized compounds using NMR spectroscopy on 1H and 13C nuclei.
- To conduct in silico screening of biological activity and acute toxicity assessment of the obtained series of substances using the PASS online and GUSAR web resources.
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EDC | 1,2-dichloroethane |
| TLC | Thin layer chromatography |
| UV | Ultraviolet |
| NMR | Nuclear magnetic resonance |
| IP | Intraperitoneal route of administration |
| IV | Intravenous route of administration |
| Oral | Oral route of administration |
| SC | Subcutaneous route of administration |
| LD50 | Median lethal dose |
| Pa | The probability the compound is active for a given activity |
| OECD | Organization for Economic Co-operation and Development |
References
- Ansari, N.; Khodagholi, F.; Amini, M. 2-Ethoxy-4,5-diphenyl-1,3-oxazine-6-one activates the Nrf2/HO-1 axis and protects against oxidative stress-induced neuronal death. Eur. J. Pharmacol. 2011, 658, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Qamar, R.; Saeed, A.; Saeed, M.; Ashraf, Z.; Abbas, Q.; Hassan, M.; Albericio, F. Synthesis, carbonic anhydrase inhibitory activity and antioxidant activity of some 1,3-oxazine derivatives. Drug Dev. Res. 2018, 79, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.P.; Kumar, A.; Sharma, S.; Shukla, P.K.; Nath, M. An eco-friendly synthesis and antimicrobial activities of dihydro-2H-benzo- and naphtho-1,3-oxazine derivatives. Eur. J. Med. Chem. 2010, 45, 1502–1507. [Google Scholar] [CrossRef] [PubMed]
- Chylińska, J.B.; Janowiec, M.; Urbański, T. Antibacterial activity of dihydro-1,3-oxazine derivatives condensed with aromatic rings in positions 5,6. Br. J. Pharmacol. 1971, 43, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Zinad, D.S.; Mahal, A.; Salman, G.A. Synthesis and Antibacterial Activity of Novel 1,3-Oxazine Derivatives. Org. Prep. Proced. Int. 2021, 53, 578–584. [Google Scholar] [CrossRef]
- Asifa, M.; Imran, M. Pharmacological Profile of Oxazine and its Derivatives: A Mini Review. Int. J. New Chem. 2020, 7, 60–73. [Google Scholar] [CrossRef]
- Zinad, D.S.; Mahal, A.; Mohapatra, R.K.; Sarangi, A.K.; Pratama, M.R.F. Medicinal chemistry of oxazines as promising agents in drug discovery. Chem. Biol. Drug Des. 2020, 95, 16–47. [Google Scholar] [CrossRef] [PubMed]
- Komarov, A.V.; Yakovlev, I.P.; Zakhs, V.E.; Prepyalov, A.V. Reaction of Phenylmalonyl Dichloride with 3-Phenylpropynamide and Transformations of the Product by the Action of Some Nucleophiles. Russ. J. Gen. Chem. 2005, 75, 770–773. [Google Scholar] [CrossRef]

| Activity | Pa |
| Immunosuppressive | 0.7 |
| Antitumor | 0.7 |
| Antieczematous | 0.7 |
| Activity | Pa |
| Immunosuppressive | 0.7 |
| Antitumor | 0.7 |
| Antieczematous | 0.4 |
| Activity | Pa |
| Anxiolytic | 0.9 |
| Fibrinolytic | 0.7 |
| Antieczematous | 0.6 |
| Activity | Pa |
| Anxiolytic | 0.8 |
| Fibrinolytic | 0.7 |
| Antieczematous | 0.8 |
| Compound | Rat IP LD50 (mg/kg) | Rat IV LD50 (mg/kg) | Rat Oral LD50 (mg/kg) | Rat SC LD50 (mg/kg) |
| 2,2′-(benzene-1,3-diyl)bis(4-hydroxy-5-methyl-6H-1,3-oxazin-6-one) | 529 | 135 | 2055 | 2705 |
| 2,2′-(benzene-1,3-diyl)bis(4-hydroxy-5-phenyl-6H-1,3-oxazin-6-one) | 860 | 143 | 3533 | 1540 |
| Diethyl 3,3′-(isophthaloylbis(azandiyloxy))bis(3-oxo-2-methylpropanoate) | 1614 | 214 | 1647 | 5616 |
| Diethyl 3,3′-(isophthaloylbis(azandiyloxy))bis(3-oxo-2-phenylpropanoate) | 1518 | 238 | 2008 | 2519 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varvarkina, A.A.; Kolesnik, D.A.; Novikova, M.P.; Yakovlev, I.P.; Levshukova, P.O. Synthesis and Biological Activity Evaluation In Silico of Bis(4-Hydroxy-6H-1,3-Oxazin-6-One) Derivatives and the Products of Their Alcoholysis. Chem. Proc. 2025, 18, 56. https://doi.org/10.3390/ecsoc-29-26712
Varvarkina AA, Kolesnik DA, Novikova MP, Yakovlev IP, Levshukova PO. Synthesis and Biological Activity Evaluation In Silico of Bis(4-Hydroxy-6H-1,3-Oxazin-6-One) Derivatives and the Products of Their Alcoholysis. Chemistry Proceedings. 2025; 18(1):56. https://doi.org/10.3390/ecsoc-29-26712
Chicago/Turabian StyleVarvarkina, Anastasia Andreevna, Denis Andreevich Kolesnik, Marina Pavlovna Novikova, Igor Pavlovich Yakovlev, and Polina Olegovna Levshukova. 2025. "Synthesis and Biological Activity Evaluation In Silico of Bis(4-Hydroxy-6H-1,3-Oxazin-6-One) Derivatives and the Products of Their Alcoholysis" Chemistry Proceedings 18, no. 1: 56. https://doi.org/10.3390/ecsoc-29-26712
APA StyleVarvarkina, A. A., Kolesnik, D. A., Novikova, M. P., Yakovlev, I. P., & Levshukova, P. O. (2025). Synthesis and Biological Activity Evaluation In Silico of Bis(4-Hydroxy-6H-1,3-Oxazin-6-One) Derivatives and the Products of Their Alcoholysis. Chemistry Proceedings, 18(1), 56. https://doi.org/10.3390/ecsoc-29-26712






