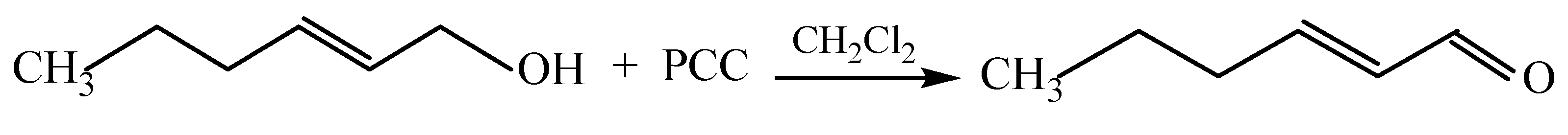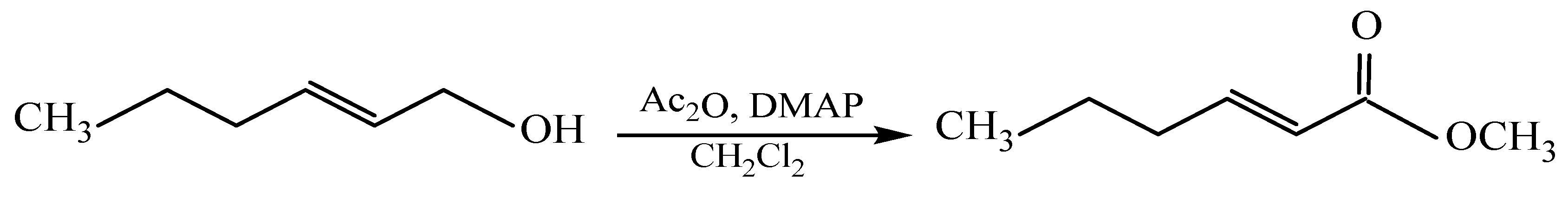1. Introduction
Stink bugs (Hemiptera: Pentatomidae and Scutelleridae) are among the most economically important insect groups, feeding on a wide range of host plants and causing serious damage to many crops [
1]. Both nymphs and adults attack wheat by feeding on leaves, stems, and grains, resulting in direct yield losses [
2].
Beyond physical feeding injury, stink bugs (Pentatomidae and Scutelleridae) secrete salivary enzymes into developing grains, initiating predigestion and enabling the insects to bypass seed defense mechanisms, including digestive enzyme inhibitors and antifeedants [
3]. These salivary secretions contain proteolytic and amylolytic enzymes, which degrade gluten proteins and starches within the grain. As a result, flour milled from damaged grains exhibits markedly reduced baking quality [
4]. Remarkably, grain damage as low as 2–3% can render an entire flour lot unacceptable for commercial baking [
5].
Frequent outbreaks of Pentatomidae and Scutelleridae in cereal crops highlight the urgent need for reliable phytosanitary assessment and the development of preventive monitoring tools. One promising approach is the identification of pheromones in Eurygaster and Aelia populations and the synthesis of highly attractive analogues. Such semiochemical tools would enable early detection of infestations and precise determination of pest distribution within different agroclimatic zones, particularly during vulnerable stages of crop development. Moreover, evaluating the influence of abiotic, biotic, and anthropogenic factors on the bioecological parameters of stink bug populations is critical for establishing sustainable pest control strategies.
Stink bugs (Pentatomidae and Scutelleridae) are known to synthesize a broad spectrum of semiochemical compounds that regulate communication and ecological interactions, including pheromones, allomones, synomones, and kairomones. Among these, protective allomones secreted by the metathoracic glands are well studied and play a central role in chemical defense. However, other semiochemically mediated behaviors, such as sexual communication, host-plant selection, and overwintering aggregation, remain insufficiently explored at the chemical and molecular levels [
6]. In our study, we identified alarm pheromones with aggregation properties from the metathoracic glands of stink bugs and, based on GC–MS structural analysis, proposed synthetic pathways for analogue compounds that can be evaluated as lures in field trials.
2. Methods and Materials
GC–MS analyses were performed on an Agilent 5000 GC with split and splitless injectors, coupled to an Agilent 5977B GC/MSD in SIM, SCAN, and EI (70 eV) modes. An HP-5 ms Ultra Inert column (30 m × 0.25 mm × 0.25 µm) was used, with hydrogen as the carrier gas (1.2 mL/min, constant flow). Injection volume was 0.25 μL in splitless mode at 280 °C. The oven temperature program was: 50 °C (1 min), 20 °C/min to 180 °C, then 0.5 °C/min to 195 °C, hold for 20 min. Transfer line, ion source, and quadrupole temperatures were set at 320 °C, 230 °C, and 150 °C, respectively. Mass spectra were acquired over the range 25–600 amu with a solvent delay of 3.5 min. Solvents and reagents were purified by standard distillation or recrystallization. TLC was carried out on Silufol silica gel plates with ethyl acetate/hexane (1:5) as the eluent, visualized with iodine vapor. Flash chromatography was performed on Merck 60 F254 silica gel using hexane/ethyl ether mixtures.
Collection of Biological Material under Field Conditions. Study of Specific Features of Maintaining Biological Material under Laboratory Conditions.
Specimens of
Eurygaster integriceps,
E. maura,
Aelia rostrata, and
A. melanota were collected from experimental wheat and barley fields. Initial field surveys involved visual inspection for insect presence, yellowing of leaves, stem necrosis, and spike wilting. Population density was estimated using systematic sampling with a 50 × 50 cm frame and entomological nets. Adults were then collected manually from foliage into glass containers. All developmental stages were present in the field, but only adult specimens were used for further study. In the laboratory, adults were maintained in rearing containers under controlled temperature and continuous ventilation. Plant material was replaced with fresh material every 2–3 days, and humidity was sustained by adding moistened filter paper to the containers (
Figure 1).
Dissection of the metathoracic glands from the biological material of Eurygaster integriceps, Eurygaster maura, Aelia rostrata, and Aelia melanota.
To prevent premature release of glandular contents, approximately 50 adult stink bugs were individually frozen prior to dissection. The insects were then fixed dorsally in Petri dishes and dissected under a stereomicroscope. Dissection involved a longitudinal incision along the dorsal abdominal margin extending to the metathoracic region and beneath the scutellum. The dorsal cuticle was gently lifted, and internal organs were removed with fine surgical scissors to expose the metathoracic glands (
Figure 2 and
Figure 3).
Following dissection, the metathoracic gland complex situated in the ventral abdominal region was carefully removed with fine forceps and placed into solvent-filled vials (hexane, methylene chloride, or dichloroethane) for subsequent chemical extraction.
Identification of the composition of metathoracic glands from the biological material, determination of structural features, and detection of dominant and minor components of the attractant substances of insect metathoracic glands.
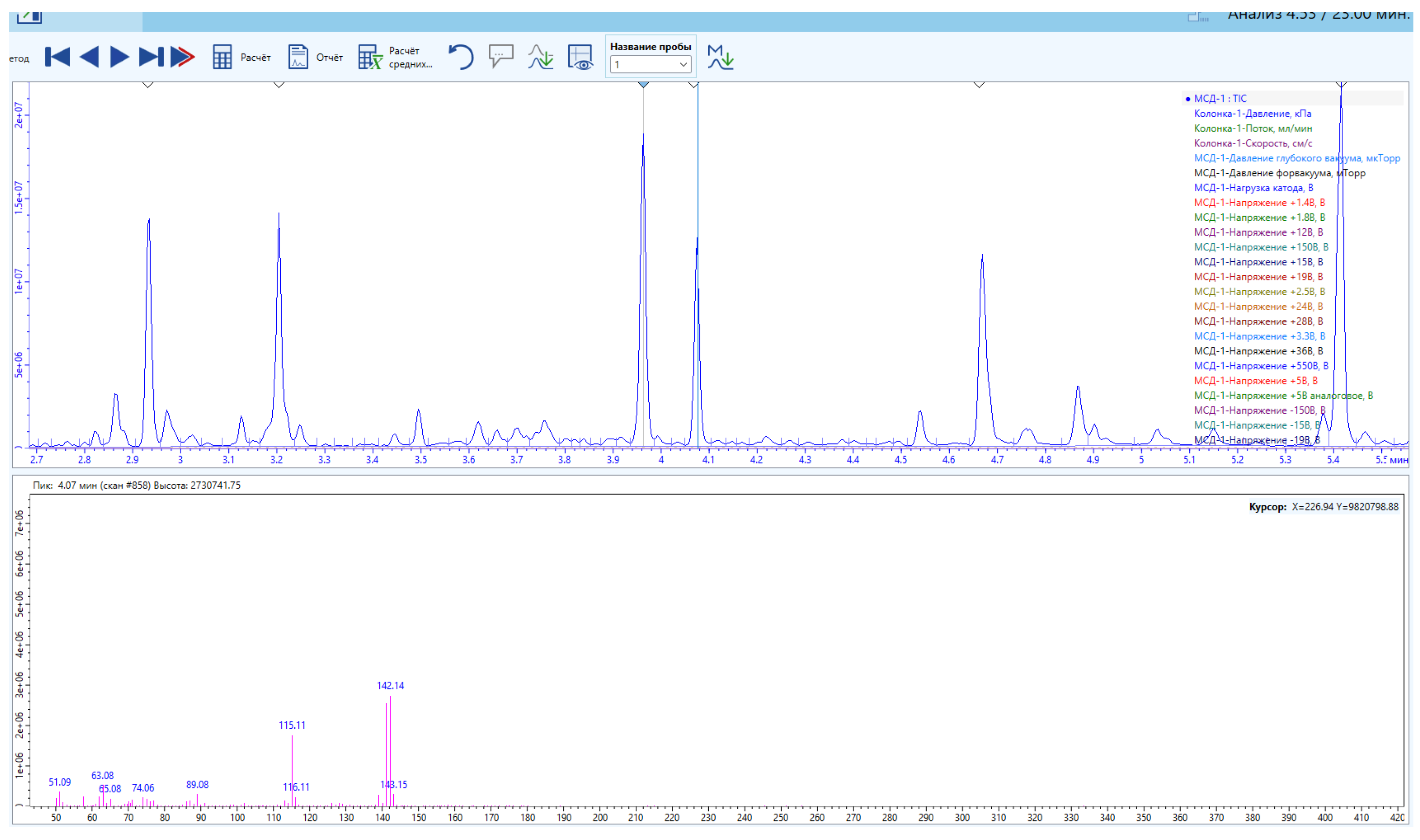
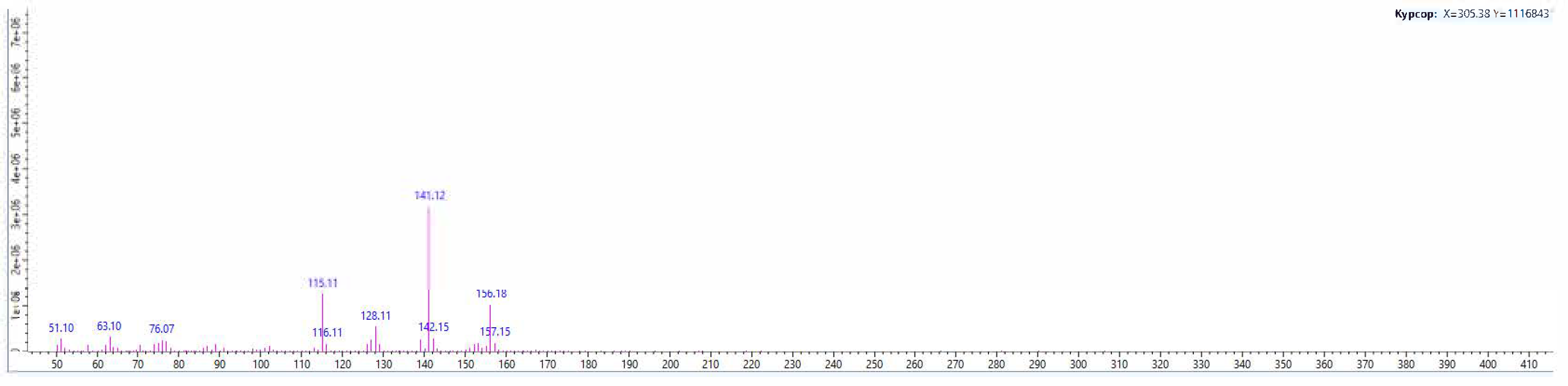
Secretions from the metathoracic glands of male and female Eurygaster integriceps, E. maura, Aelia rostrata, and A. melanota were analyzed by GC–MS, and the results are presented in
Figure 4 and
Figure 5.
Preliminary GC–MS results indicated that the metathoracic gland extracts of all examined species contained identical major constituents, specifically (E)-2-hexen-1-ol acetate and n-undecane. Both compounds are characterized by strong odors and irritant properties, making them effective chemical defenses that function as readily detectable warning signals. Furthermore, the hydrocarbon fraction plays an important role in modulating semiochemical release, acting as both a solvent and a substrate for the controlled emission of more volatile compounds.
Different synthetic pathways for the obtaining pheromone components and key synthons.
Synthesis of (E)-2-hexenal. (E)-2-hexen-1-ol (5.0 g, 0.05 mol) dissolved in 25 mL of methylene chloride was added dropwise to a suspension of pyridinium chlorochromate (PCC, 13.0 g, 0.06 mol) in 100 mL of methylene chloride. The reaction mixture gradually turned black, consistent with aldehyde formation, and was stirred at room temperature for 3 h. After the addition of 50 mL of hexane, stirring was continued for 30 min. The crude product was purified by flash chromatography on silica gel (SiO2, L100/160) with methylene chloride/hexane (2:1) as the eluent, yielding 3.0 g (65%) of (E)-2-hexenal.
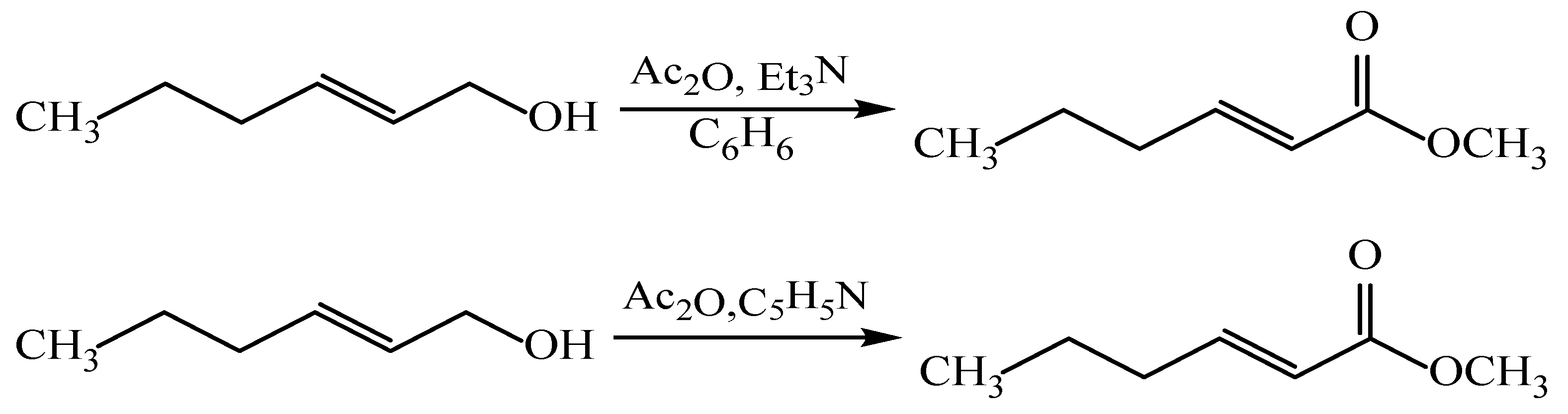
Synthesis of (E)-2-hexen-1-yl acetate (Method 1). A solution of (E)-2-hexen-1-ol (0.05 mol) and acetic anhydride (0.05 mol) in benzene (100 mL) was prepared in a round-bottom flask equipped with mechanical stirring and a reflux condenser fitted with a CaCl2 drying tube. One drop of concentrated sulfuric acid was added as a catalyst, and the reaction mixture was refluxed with continuous stirring for 6 h. After completion, the mixture was poured onto ice, and the organic phase was separated. The organic layer was washed with aqueous sodium carbonate solution (3 × 50 mL) followed by water (3 × 50 mL), then dried over anhydrous Na2SO4. The solvent was evaporated under reduced pressure, and the crude product was purified by vacuum distillation (bp 85–87 °C at 1 mmHg), affording 4.5 g (65%) of (E)-2-hexen-1-yl acetate.
Synthesis of (E)-2-hexen-1-yl acetate (Method 2). A mixture of (E)-2-hexen-1-ol (5.0 g, 0.05 mol) and acetic anhydride (10.2 mL, 0.1 mol) was stirred at room temperature in the presence of pyridine (15.0 mL) for 15 h. The reaction was quenched by pouring onto ice-cold water (~100 mL), and the mixture was extracted with diethyl ether (3 × 25 mL). The organic phase was separated, washed with water (50 mL) and then with saturated NaCl solution (50 mL), and dried over anhydrous Na2SO4. Following solvent removal, the crude product was purified by vacuum distillation to afford (E)-2-hexen-1-yl acetate (bp 85–87 °C/1.0 mmHg, yield 5.5 g, 70%).
Synthesis of (E)-2-hexen-1-yl acetate (DMAP-catalyzed). (E)-2-hexen-1-ol (0.95 mmol) was reacted with acetic anhydride (0.95 mmol) in the presence of DMAP (0.095 mmol) as a catalyst in methylene chloride, and the mixture was stirred for 30 min at room temperature. The reaction was diluted with methylene chloride (10 mL) and quenched with 30% aqueous acetic acid until the pH reached 5–6. The organic layer was separated, washed with saturated NaCl solution, and dried over anhydrous Na2SO4. After solvent removal under reduced pressure, the crude product was purified by flash chromatography on silica gel (SiO2, L100/160) using hexane/ether (1:1) as the eluent (Rf = 0.8). The reaction afforded the acetate in 90% crude yield with >95% purity confirmed by GC.
3. Results and Discussion
The use of pheromones in hemipteran pest management has considerable potential, but their application must be evaluated on a case-by-case basis, taking into account both biological characteristics and economic feasibility. Comparable research on termites demonstrated that bait-based systems can be optimized as a cost-effective control strategy. Hemipteran responses to pheromones vary markedly among species. For instance, mirids of the genus
Phytocoris respond strongly to synthetic pheromone analogues, and pheromone traps are widely employed for monitoring their population dynamics and associated crop damage. In contrast, phytophagous stink bugs in the families Pentatomidae and Scutelleridae are only weakly attracted to pheromone sources when other communication cues are absent [
7]. These insects often approach pheromone sources but fail to enter traps, as short-range orientation relies primarily on vibrational signals rather than volatile chemicals. Consequently, mating in Pentatomidae and Scutelleridae involves a two-step process: long-range attraction mediated by pheromones and short-range localization facilitated by substrate-borne vibratory signals generated by both sexes [
8].
Many species of stink bugs are polyphagous, exhibit high mobility, and readily disperse between cultivated crops and adjacent vegetation. Owing to their wide host range and capacity for rapid movement, they are considered one of the four most economically significant insect pest groups globally [
9]. A key adaptive feature of these insects is the presence of well-developed scent glands [
10,
11], which secrete a variety of defensive compounds that deter predators. These secretions typically contain short-chain alcohols, aldehydes, esters, (E)-2-alkenals, 4-oxo-(E)-2-alkenals, alkanes, monoterpenes, and aromatic alcohols and aldehydes. The chemistry of stink bug pheromones is even more diverse, encompassing simple esters, monoterpenes, linear, monocyclic, and multicyclic sesquiterpenoids, as well as structurally novel acetogenins.
For many Pentatomidae and Scutelleridae species, pheromone-based mating disruption strategies are limited in effectiveness because the insects do not remain consistently on a given crop; after mating, females readily migrate to other host plants. Identified pheromone components range from relatively simple straight-chain esters and aldehydes to structurally more complex molecules. However, the biological role of these semiochemicals is still poorly characterized. In certain species, sex pheromones are unambiguous, with one sex releasing the signal and the other responding to facilitate mating. In contrast, in species where adults of both sexes, and even immature nymphs, respond to the same compounds, the functional basis of this attraction remains unresolved. Clarifying these communication mechanisms is critical for assessing the feasibility and reliability of pheromone-based tools in pest management programs targeting Pentatomidae and Scutelleridae.
Monitoring stink bug populations in cereal crops through conventional entomophytosanitary methods is time-consuming and laborious, often requiring repetitive field sweeping, laboratory sorting, and detailed species-level identification—tasks made even more challenging under conditions of high pest density. This has created a need for alternative, more objective approaches to hemipteran monitoring. According to published studies, the main semiochemical attractants of stink bugs are 2-alkenals (particularly E-isomers), saturated aliphatic hydrocarbons, and 4-oxo-(E)-2-alkenals [
12]. Pheromone structures have been elucidated for nearly 45 species of Pentatomidae and Scutelleridae [
13], with research efforts concentrated primarily on pests of soybean, coffee, and fruit and vegetable crops. The chemical ecology of stink bugs in this region remains largely uncharacterized. By contrast, pheromonal communication in stink bugs infesting cereal crops in Uzbekistan has not previously been examined. Our study addresses this gap, and we expect that results from field trials will contribute to the development of pheromone-based monitoring tools for integration into cereal crop pest management programs.
Development of Synthetic Methods for Analogues of Attractive Compounds of Stink Bugs.
Stink bugs are often described as miniature chemical factories due to their well-developed scent glands, which occur in the abdomen of immature stages and in the metathoracic region of adults [
14]. In many phytophagous Pentatomidae and Scutelleridae, pheromones differ both chemically and functionally from defensive secretions and are synthesized in tissues separate from those producing defensive compounds. Nonetheless, the distinction between pheromonal and defensive secretions has sometimes been blurred. A number of compounds have been designated as pheromones despite minimal or inconclusive bioassay data confirming their function as intraspecific signals involved in mating, aggregation, or other behaviors.
Previous studies have demonstrated that α,β-unsaturated aldehydes can act simultaneously as aggregation pheromones, alarm signals, and defensive secretions in insects [
15,
16]. Experimental evidence indicates that exposure to C6–C8 α,β-unsaturated aldehydes produces moderate but reversible paralysis in crickets. This phenomenon has been attributed to (E)-2-alkenals, whose relatively small molecular size and structural features facilitate rapid cuticular penetration. Conversely, aldehydes with longer carbon chains exhibit reduced penetration efficiency. Similar effects were observed for (E)-2-hexen-1-ol, which also induced temporary locomotor paralysis. These findings suggest that short-chain α,β-unsaturated compounds may interfere with locomotion-related physiological processes, potentially by disrupting neurotransmission through competitive antagonism or weak blockade of neuronal target sites.
Analysis of metathoracic gland secretions from
Eurygaster integriceps,
E. maura,
Aelia rostrata, and
A. melanota identified (E)-2-hexen-1-ol acetate as the dominant compound. To support further applications, we optimized its synthesis and proposed alternative synthetic routes using improved reagents and catalytic techniques. Literature indicates that α,β-unsaturated aldehydes, including (E)-2-hexenal, play multiple ecological roles as aggregation pheromones, alarm signals, and defensive compounds [
17]. Their biological activity is partly due to electrophilic reactivity, enabling covalent interactions with cysteine residues in proteins, enzyme active sites, glutathione, and other thiol-containing molecules such as coenzyme A, which can disrupt physiological processes and act as a toxic defense mechanism [
18,
19,
20]. In our study, (E)-2-hexen-1-ol acetate, together with n-undecane and n-tridecane, represented approximately 90% of the secretion profile and were consistently detected in both
Aelia and
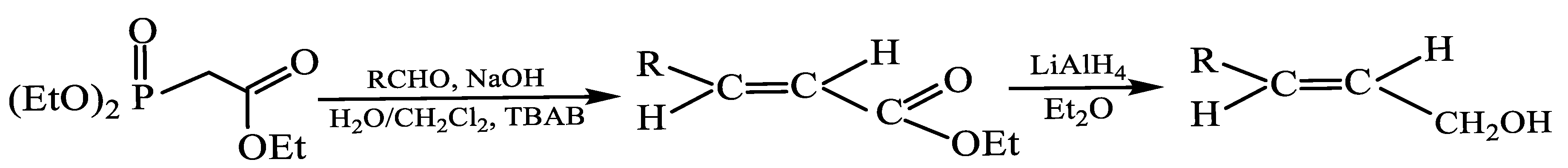
Eurygaster. These long-chain hydrocarbons likely act as solvents or carriers, regulating the release of more volatile semiochemicals. Building on these structural insights, we developed an original method for synthesizing analogues of the identified compounds, which will be evaluated in field trials for their attractiveness to stink bugs. Unsaturated E-fragment compounds were synthesized via the Wittig–Horner reaction under phase-transfer catalytic conditions, providing efficient access to the desired products in high yield. Comparable approaches have been applied in the synthesis of weevil aggregation pheromone analogues [
21].
(E)-2-hexen-1-ol and (E)-5-decen-1-ol were synthesized under the described conditions, affording 55% yield
Scheme 1. The obtained (E)-2-hexen-1-ol was subsequently oxidized with pyridinium chlorochromate (PCC), producing (E)-2-hexenal in 75% yield, as outlined in
Scheme 2.
In addition, the unsaturated alcohol was acetylated under standard reaction conditions to give the corresponding acetate in 65% yield, as presented in
Scheme 3.
The resulting unsaturated alcohol was also converted into the corresponding acetate in the presence of dimethylaminopyridine (DMAP) as a catalyst [
14], using acetic anhydride.
Catalytic conditions significantly improved the synthetic process, reducing the reaction time to 30 min and increasing the yield to 90%
Scheme 4. This approach thus demonstrated clear advantages for obtaining the desired product efficiently.
Comprehensive GC–MS analysis of metathoracic gland extracts enabled the identification and structural characterization of both dominant and minor secretion components. The major compounds detected were (E)-2-hexen-1-ol acetate and n-undecane, which together constituted approximately 90% of the total extract content and were found in both sexes. These compounds were consistently associated with Aelia rostrata, A. melanota, Eurygaster integriceps, and E. maura, species that are widespread in cereal-growing regions of Uzbekistan and Central Asia.
On the basis of structural analysis, we designed synthetic methods for the preparation of pheromone precursors (synthons) and successfully synthesized the major semiochemical components intended for application in pheromone-based monitoring systems.
Alongside the dominant compounds, additional substances were identified in both male and female specimens, including (E)-2-hexenal, (E)-5-decen-1-ol, n-tridecane, hexadecane, and tridecyl butyrate. These aldehydes and esters are characterized by strong odors and irritant properties, enabling them to function as warning signals and defensive secretions. The long-chain hydrocarbons are likely involved in modulating semiochemical release, serving as substrates for the gradual emission of more volatile compounds. Taken together, these findings indicate that the metathoracic gland secretions include alarm pheromones with aggregation effects.