Insilico Evaluation of Chrome-4-One Derivatives as a Potential α-Glucosidase Inhibitor: Molecular Docking and ADMET Profiling †
Abstract
1. Introduction
2. Methods
2.1. Ligand Preparation
2.2. Receptor Preparation
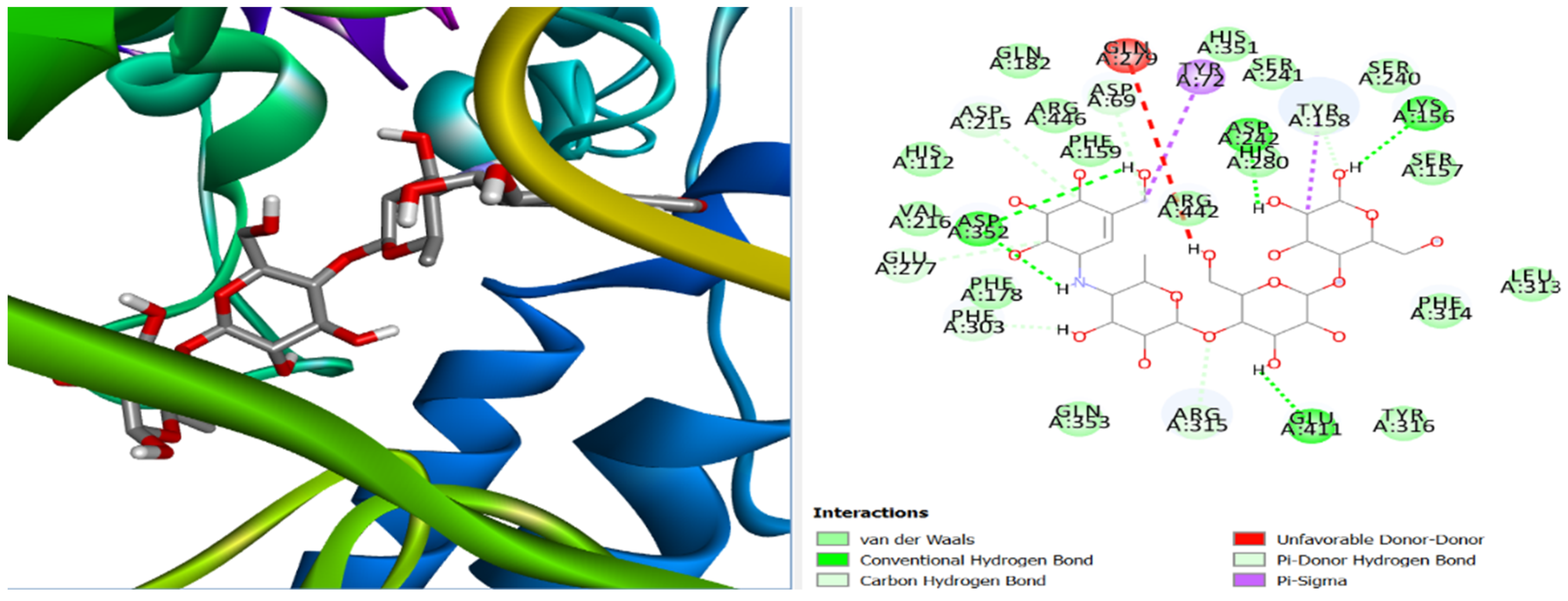
2.3. Molecular Docking
2.4. Theoretical Oral Bioavailability
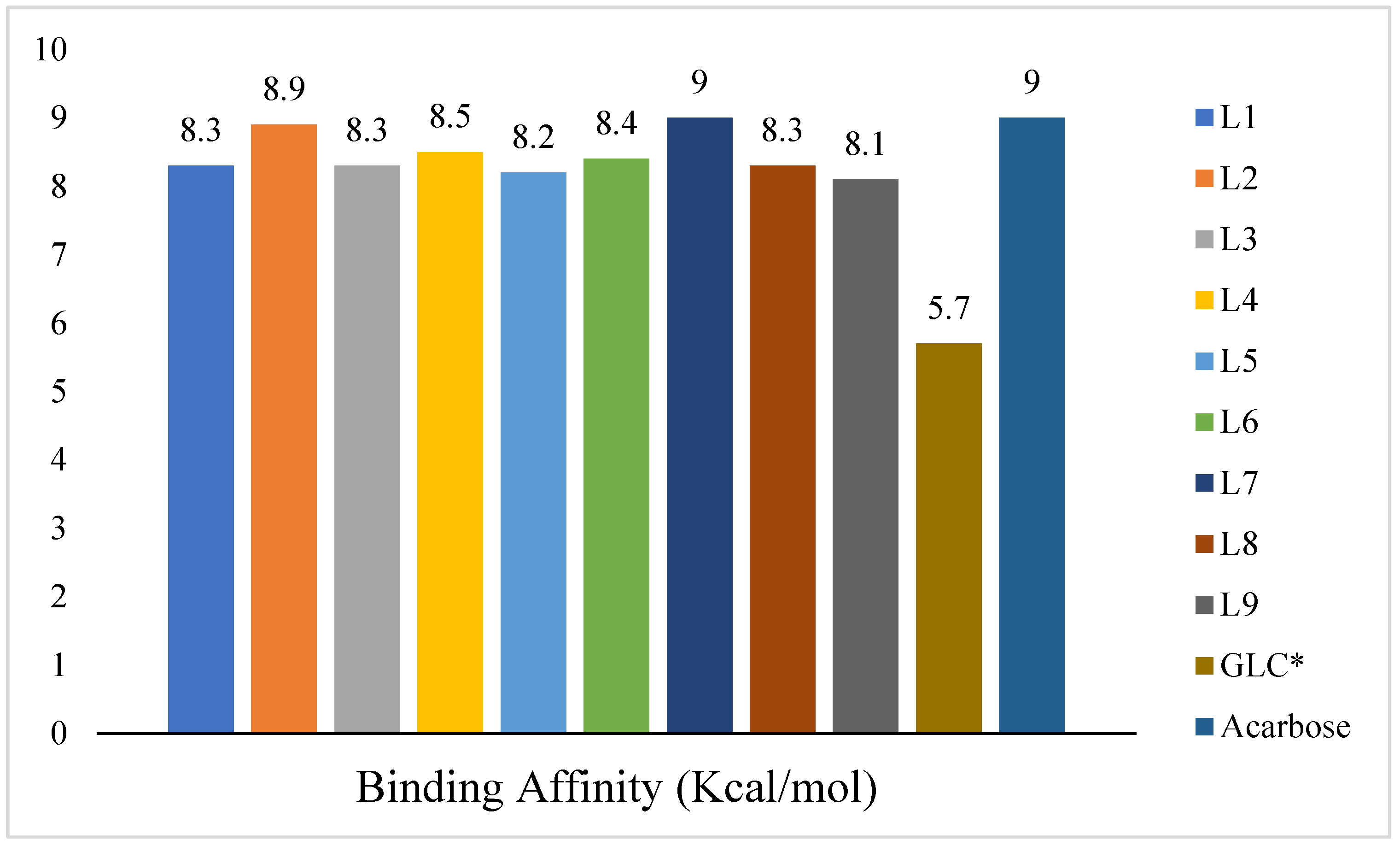
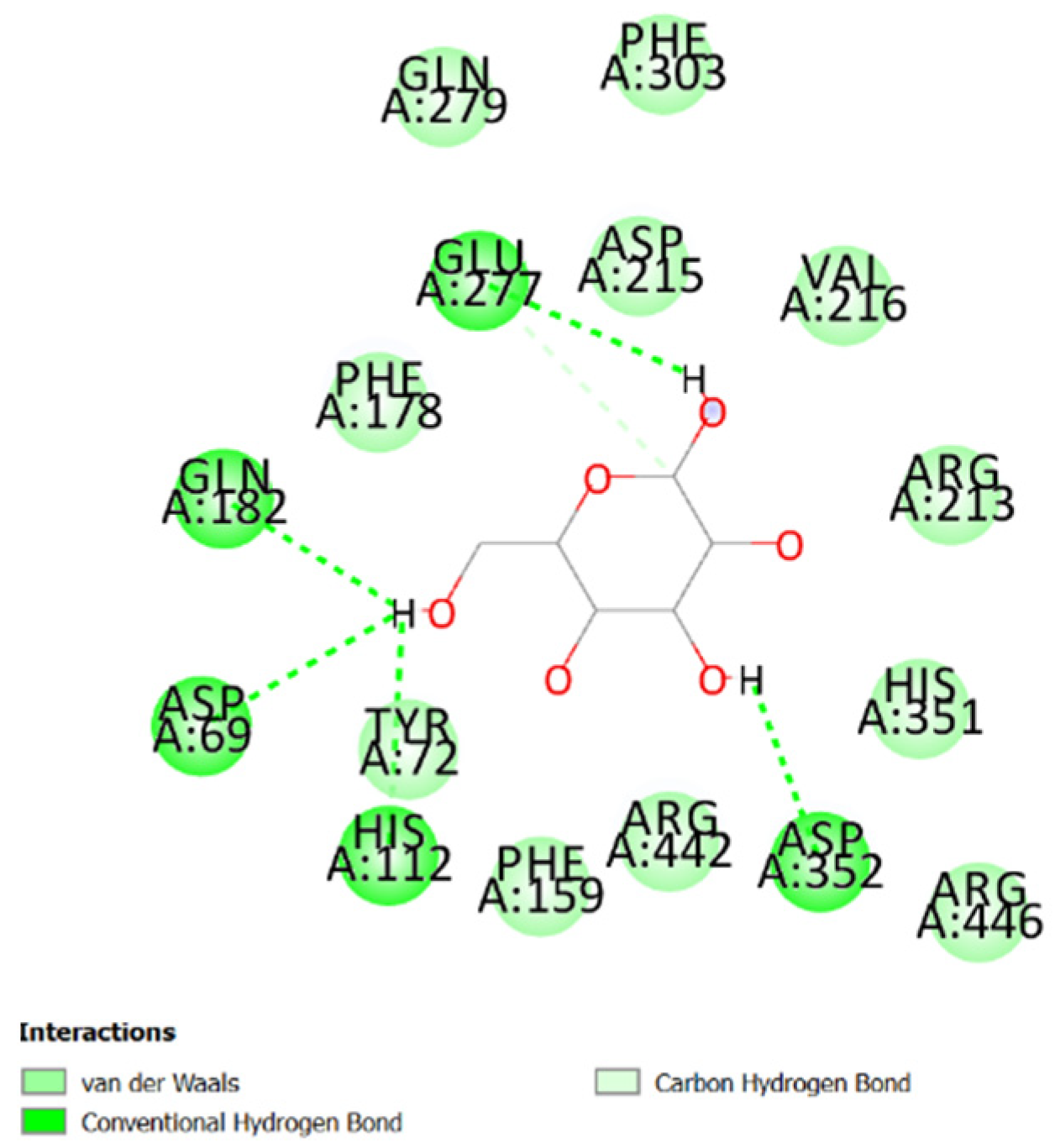
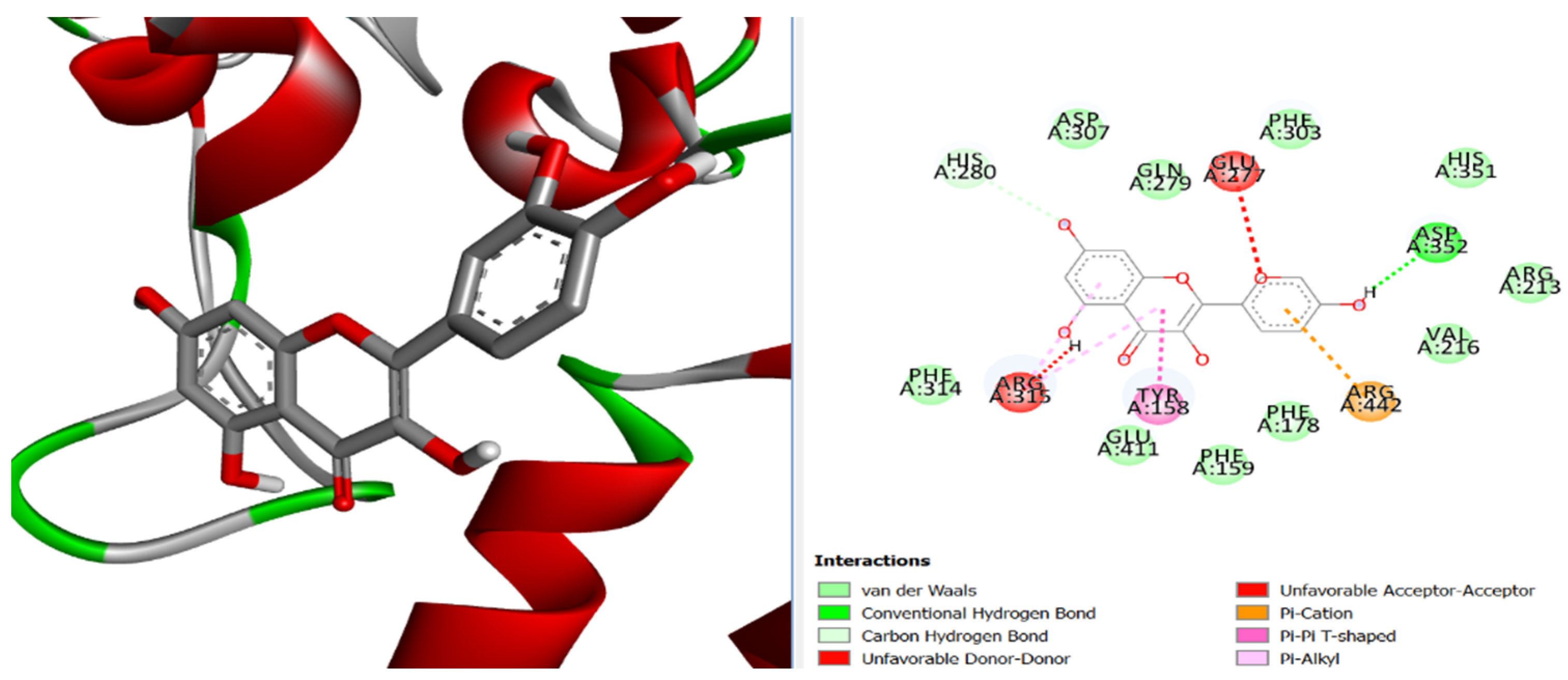
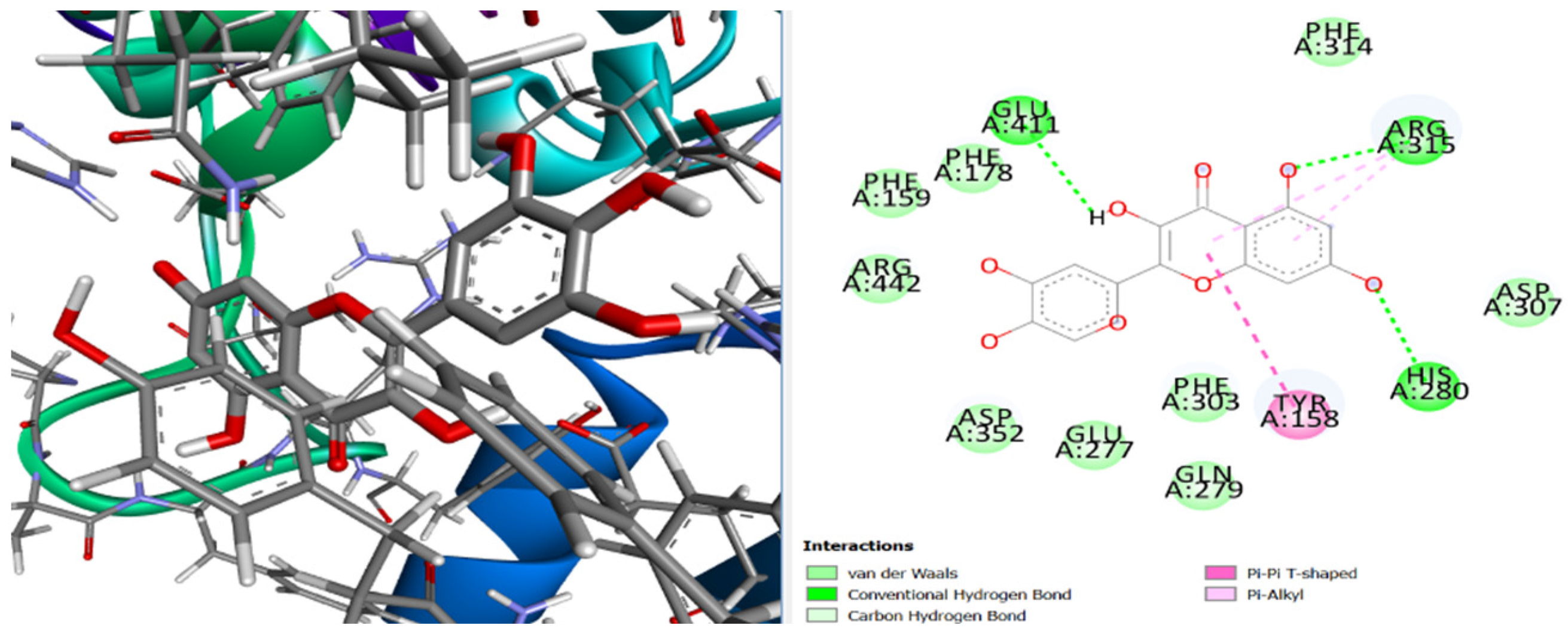
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 2009, 37, S62–S67. [Google Scholar]
- Mendes, A.L.; Miot, H.A.; Junior, V.H. Diabetes mellitus and the skin. An Bras. Dermatol. 2017, 92, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Kumara, S.T.; Patrudu, T.B.; Polisetti, V.K.; Chinnachennaiahgari, V.B.; Yatam, S.; Katari, N.K.; Gundla, R. Dihydropyrimidinone-Based Chromones as New α-Glucosidase Inhibitors. ChemistrySelect 2025, 10, e05463. [Google Scholar] [CrossRef]
- Padhi, S.; Kumar, A.; Behera, A. Biomedicine & pharmacotherapy type II diabetes mellitus: A review on recent drug-based therapeutics. Biomed. Pharmacother. 2020, 131, 110708. [Google Scholar] [PubMed]
- Dirir, A.; Daou, M.; Yousef, A.; Yousef, L. A review of alpha-glucosidase inhibitors from plants as potential candidates for the treatment of type-2 diabetes. Phytochem. Rev. 2021, 21, 1049–1079. [Google Scholar] [CrossRef]
- Kuldeep, T.P.; Santosh, S.C.; Manoj Damale Jaiprakash, N.S.; Navanand, B.W.; Gokul, V.S.; Ramesh, S.N.; Sunil, V.G. Design, synthesis, and pharmacological profiling of 2-(Furan-2-yl)- chromen-4-one derivatives: Theoretical and molecular insights. Results Chem. 2025, 17, 102588. [Google Scholar] [CrossRef]
- Benny, A.T.; Arikkatt, S.D.; Vazhappilly, C.G.; Kannadasan, S.; Leelabaiamma, M.S.N.; Thomas, R.; Radha, E.K.; Shanmugam, P. Chromone, A Privileged Scaffold in Drug Discovery: Developments in the Synthesis and Bioactivity. Mini-Rev. Med. Chem. 2022, 22, 1030–1063. [Google Scholar] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Sanner, M.F. Python: A Programming Language for Software Integration and Development. J. Mol. Graph. Mod. 1999, 17, 57–61. [Google Scholar]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]

| Common Name | Code | PubChem ID | IUPAC Name | Chemical Structure | SMILES |
|---|---|---|---|---|---|
| Genistein | L1 | 5280961 | 5,7-dihydroxy-3-(4-hydroxyphenyl)chromen-4-one |  | C1=CC(=CC=C1C2=COC3=CC(=CC(=C3C2=O)O)O)O |
| Quercetin | L2 | 5280343 | 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxychromen-4-one | 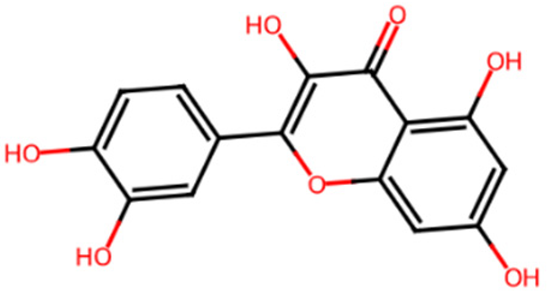 | C1=CC(=C(C=C1C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O)O |
| Apigenin | L3 | 5280443 | 5,7-dihydroxy-2-(4-hydroxyphenyl)chromen-4-one |  | C1=CC(=CC=C1C2=CC(=O)C3=C(C=C(C=C3O2)O)O)O |
| Diosmetin | L4 | 5281612 | 5,7-dihydroxy-2-(3-hydroxy-4-methoxyphenyl)chromen-4-one | 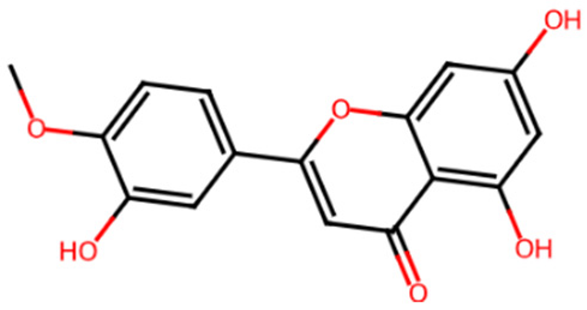 | COC1=C(C=C(C=C1)C2=CC(=O)C3=C(C=C(C=C3O2)O)O)O |
| Tangeretin | L5 | 68077 | 5,6,7,8-tetramethoxy-2-(4-methoxyphenyl)chromen-4-one |  | COC1=CC=C(C=C1)C2=CC(=O)C3=C(O2)C(=C(C(=C3OC)OC)OC)OC |
| kaempferol | L6 | 5280863 | 3,5,7-trihydroxy-2-(4-hydroxyphenyl)chromen-4-one |  | C1=CC(=CC=C1C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O)O |
| Myricetin | L7 | 5281672 | 3,5,7-trihydroxy-2-(3,4,5-trihydroxyphenyl)chromen-4-one | 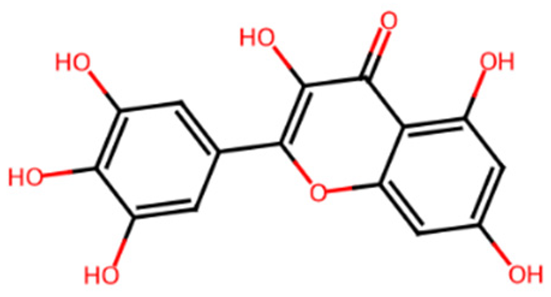 | C1=C(C=C(C(=C1O)O)O)C2=C(C(=O)C3=C(C=C(C=C3O2)O)O)O |
| Daidzein | L8 | 5281708 | 7-hydroxy-3-(4-hydroxyphenyl)chromen-4-one |  | C1=CC(=CC=C1C2=COC3=C(C2=O)C=CC(=C3)O)O |
| Flavone | L9 | 10680 | 2-phenylchromen-4-one |  | C1=CC=C(C=C1)C2=CC(=O)C3=CC=CC=C3O2 |
| S/No | Code | Mw (g/mol) | GI Absorption | LogP | n-HA | n-HD | SA Score | Lipinski’s Violation | Inference |
|---|---|---|---|---|---|---|---|---|---|
| 1 | L1 | 270 | High | 2.07 | 5 | 3 | 2.0 | 0 | Pass |
| 2 | L2 | 302 | High | 1.45 | 7 | 5 | 2.0 | 0 | Pass |
| 3 | L3 | 270 | High | 2.98 | 5 | 3 | 2.0 | 0 | Pass |
| 4 | L4 | 300 | High | 2.63 | 6 | 3 | 2.0 | 0 | Pass |
| 5 | L5 | 372 | High | 2.45 | 7 | 0 | 2.0 | 0 | Pass |
| 6 | L6 | 286 | High | 1.97 | 6 | 4 | 2.0 | 0 | Pass |
| 7 | L7 | 318 | High | 1.12 | 8 | 6 | 2.0 | 1 | Pass |
| 8 | L8 | 254 | High | 2.22 | 4 | 2 | 2.0 | 0 | Pass |
| 9 | L9 | 222 | High | 3.80 | 2 | 0 | 2.0 | 0 | Pass |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gidado, I.; Bello, A.S.; Gatugel, Y.A.; Ibrahim, M.; Inuwa, Y. Insilico Evaluation of Chrome-4-One Derivatives as a Potential α-Glucosidase Inhibitor: Molecular Docking and ADMET Profiling. Chem. Proc. 2025, 18, 51. https://doi.org/10.3390/ecsoc-29-26856
Gidado I, Bello AS, Gatugel YA, Ibrahim M, Inuwa Y. Insilico Evaluation of Chrome-4-One Derivatives as a Potential α-Glucosidase Inhibitor: Molecular Docking and ADMET Profiling. Chemistry Proceedings. 2025; 18(1):51. https://doi.org/10.3390/ecsoc-29-26856
Chicago/Turabian StyleGidado, Ibrahim, Abubakar Sadiq Bello, Yusuf Adamu Gatugel, Modu Ibrahim, and Yusuf Inuwa. 2025. "Insilico Evaluation of Chrome-4-One Derivatives as a Potential α-Glucosidase Inhibitor: Molecular Docking and ADMET Profiling" Chemistry Proceedings 18, no. 1: 51. https://doi.org/10.3390/ecsoc-29-26856
APA StyleGidado, I., Bello, A. S., Gatugel, Y. A., Ibrahim, M., & Inuwa, Y. (2025). Insilico Evaluation of Chrome-4-One Derivatives as a Potential α-Glucosidase Inhibitor: Molecular Docking and ADMET Profiling. Chemistry Proceedings, 18(1), 51. https://doi.org/10.3390/ecsoc-29-26856







