Synthesis and Characterization of 6-(1,3-dimethylureido) dibenzo[c,e][1,2]oxaphosphinine 6-oxide †
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
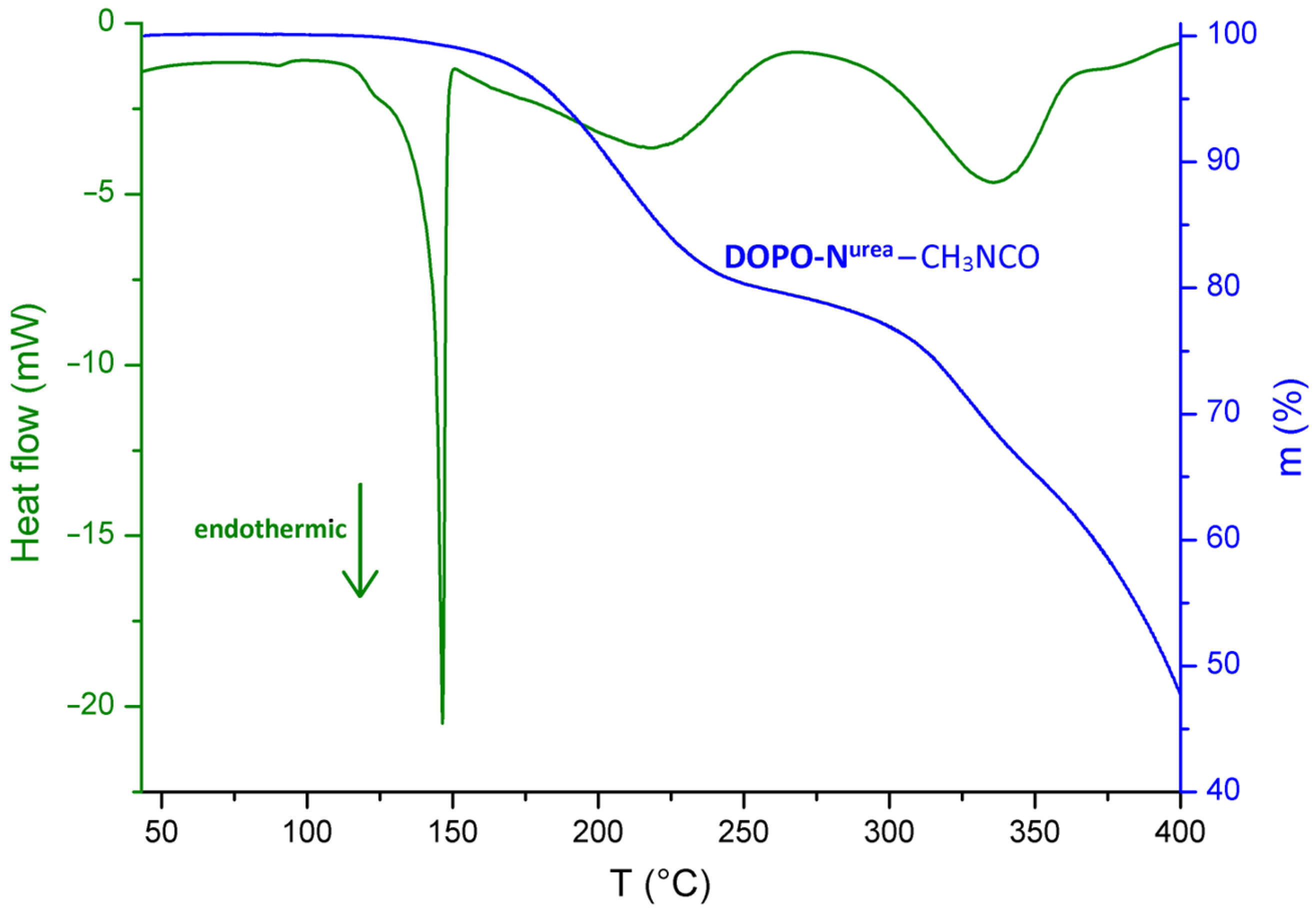
2.2. Characterizations
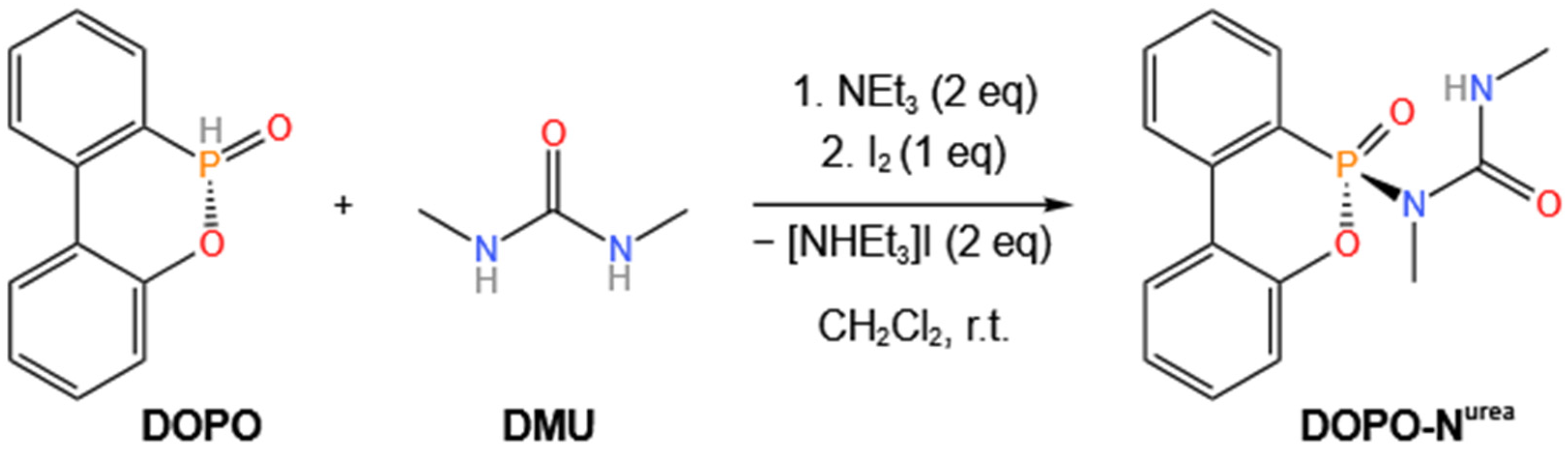
2.3. Synthesis of DOPO-Nurea
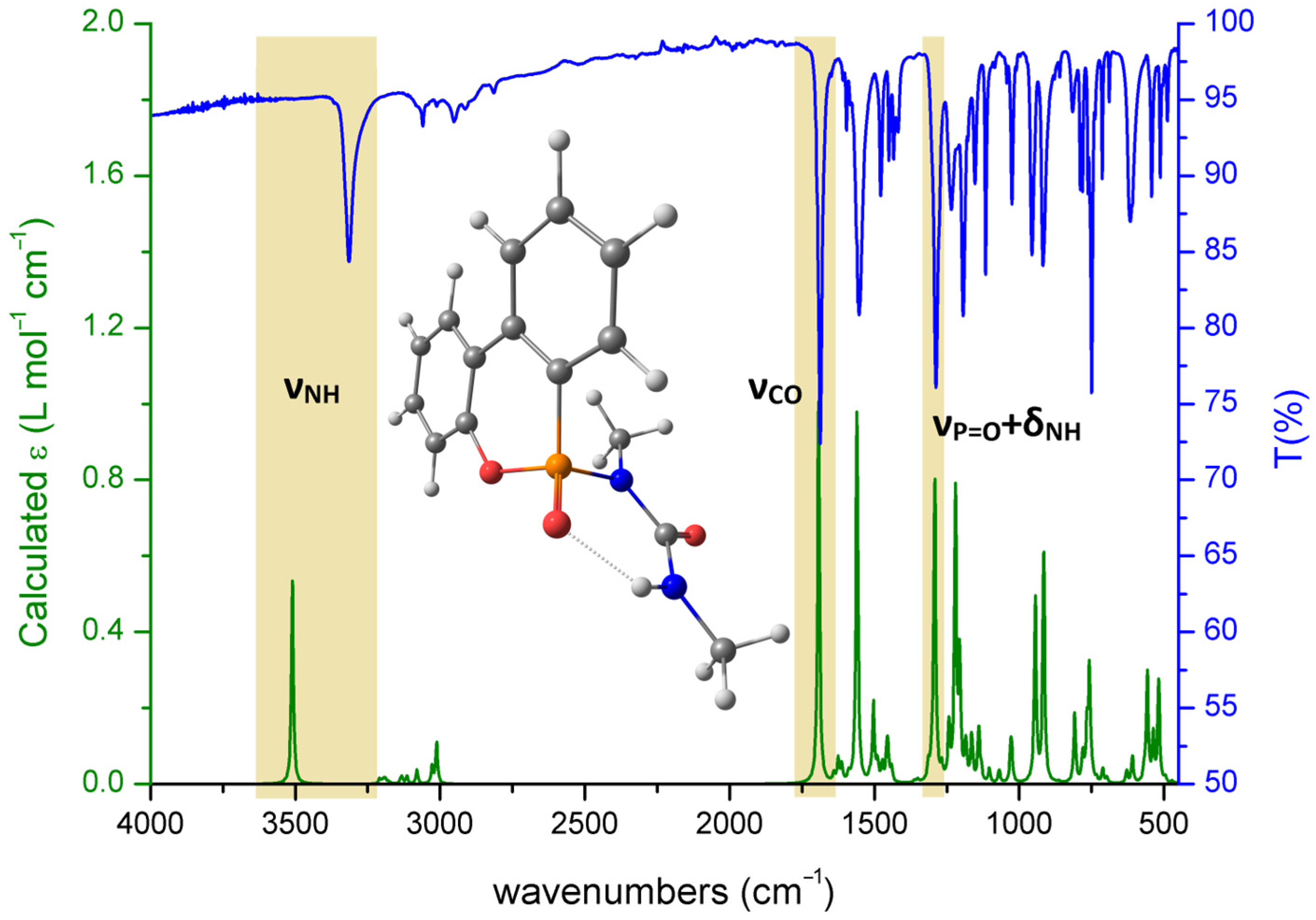
2.4. Computational Simulations
3. Results and Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pack, S. A Review of Non-halogen Flame Retardants in Epoxy-Based Composites and Nanocomposites: Flame Retardancy and Rheological Properties. In Flame Retardants; Visakh, P.M., Arao, Y., Eds.; Springer: Heidelberg, Germany, 2015; pp. 115–130. [Google Scholar]
- Marosi, G.; Szolnoki, B.; Bocz, K.; Toldy, A. Reactive and Additive Phosphorus-based Flame Retardants of Reduced Environmental Impact. In Polymer Green Flame Retardants; Papaspyrides, C.D., Kiliaris, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 181–220. [Google Scholar]
- Olcay, H.; Gul, C.; Kocak, D.E. Synergism in Nitrogen- and Phosphorus-Based Flame Retardants. In Materials and Chemistry of Flame-Retardant Polyurethanes—Volume 2: Green Flame Retardants; Gupta, R.K., Ed.; ACS Symposium Series: Washington, DC, USA, 2021; pp. 213–247. [Google Scholar]
- Wagner, S.; Rakotomalala, M.; Bykov, Y.; Walter, O.; Döring, M. Synthesis of new organophosphorus compounds using the Atherton–Todd reaction as a versatile tool. Heteroatom. Chem. 2012, 23, 216–222. [Google Scholar] [CrossRef]
- Gaan, S.; Neisius, M.; Mercoli, P.; Liang, S.; Mispreuve, H.; Näscher, R. Phosphonamidates-Synthesis and Flame Retardant Applications. U.S. Patent 9650497B2, 16 May 2017. [Google Scholar]
- Jian, R.; Wang, P.; Duan, W.; Wang, J.; Zheng, X.; Weng, J. Synthesis of a Novel P/N/S-Containing Flame Retardant and Its Application in Epoxy Resin: Thermal Property, Flame Retardance, and Pyrolysis Behavior. Ind. Eng. Chem. Res. 2016, 55, 11520–11527. [Google Scholar] [CrossRef]
- Gaan, S.; Neisius, M.; Mercoli, P.; Liang, S.; Mispreuve, H.; Näscher, R. Novel Phosphonamidates-Synthesis and Flame Retardant Application. WIPO WO2013020696A3, 10 May 2013. [Google Scholar]
- Neisius, N.M.; Lutz, M.; Rentsch, D.; Hemberger, P.; Gaan, S. Synthesis of DOPO-Based Phosphonamidates and their Thermal Properties. Ind. Eng. Chem. Res. 2014, 53, 2889–2896. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Baumgartner, G.; Jovic, M.; Gössi, A.; Riedl, W.; Zich, T.; Gaan, S. Industrial Upscaling of DOPO-Based Phosphonamidates and Phosphonates Derivatives Using Cl2 Gas as a Chlorinating Agent. Org. Process Res. Dev. 2018, 22, 1570–1577. [Google Scholar] [CrossRef]
- Salmeia, K.A.; Flaig, F.; Rentsch, D.; Gaan, S. One-Pot Synthesis of P(O)-N Containing Compounds Using N-Chlorosuccinimide and Their Influence in Thermal Decomposition of PU Foams. Polymers 2018, 10, 740. [Google Scholar] [CrossRef] [PubMed]
- Tada, Y.; Sunada, A.; Watanabe, R.; Kanazawa, M.; Utsumi, K. Synthesis of Trispirocyclotriphosphazenes with Oxaphosphorine Rings and Their Crystal and Molecular Structures. Inorg. Chem. 2021, 60, 5014–5020. [Google Scholar] [CrossRef] [PubMed]
- Kukla, P.; Greiner, L.; Eibl, S.; Döring, M.; Schönberger, F. Novel phosphorus-containing silazanes as flame retardants in epoxy resins. React. Funct. Polym. 2022, 170, 105120. [Google Scholar] [CrossRef]
- Marra, G.; Bortoluzzi, M.; Agostinis, L. One-Pot Synthesis of Phosphoramidates from dibenzo[1,3,2]dioxaphosphepine-6-oxide. Chem. Proc. 2023, 14, 18. [Google Scholar] [CrossRef]
- Bortoluzzi, M.; Ghincolov, S.; Agostinis, L. Alternative Synthesis of Phosphonate Derivatives of 9,10-Dihydro-9-oxa-10-phosphaphenanthrene-10-oxide. Chem. Proc. 2023, 14, 19. [Google Scholar] [CrossRef]
- Gagliardi, V.; Castro, J.; Beghetto, V.; Expósito, M.; Bortoluzzi, M. Luminescence of the Conjugate Bases of [2-(2-Hydroxyphenyl)phenyl]phosphinic Acid and Single-Crystal X-Ray Structure Determination of Sodium [2-(2-Hydroxyphenyl)phenyl]phosphinate. Organics 2025, 6, 10. [Google Scholar] [CrossRef]
- Ferraro, V.; Castro, J.; Agostinis, L.; Bortoluzzi, M. Dual-emitting Mn(II) and Zn(II) halide complexes with 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide as ligand. Inorg. Chim. Acta 2023, 545, 121285. [Google Scholar] [CrossRef]
- Agostinis, L.; Ghincolov, S.; Bortoluzzi, M. Preparation process of P(=O)-heteroatom derivatives of dibenzooxaphosphacycles. WIPO WO2023094526A1, 1 June 2023. [Google Scholar]
- Bortoluzzi, M.; Agostinis, L.; Ghincolov, S.; Ferraro, V.; Marra, G.; Castro, J. Straightforward synthesis of 9,10-dihydro-9-oxa-10-phosphaphenanthrene-10-oxide derivatives containing P–N bonds. Chem. Pap. 2023, 77, 7999–8006. [Google Scholar] [CrossRef]
- Grimme, S.; Hansen, A.; Ehlert, S.; Mewes, J.-M. r2SCAN-3c: A “Swiss army knife” composite electronic-structure method. J. Chem. Phys. 2021, 154, 064103. [Google Scholar] [CrossRef] [PubMed]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comput. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef] [PubMed]
- Neese, F. Software update: The ORCA program system–Version 5.0. WIREs Comput. Mol. Sci. 2022, 12, e1606. [Google Scholar] [CrossRef]
- Yang, L.; Powell, D.R.; Houser, R.P. Structural variation in copper(I) complexes with pyridylmethylamide ligands: Structural analysis with a new four-coordinate geometry index, τ4. Dalton Trans. 2007, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Honorien, J.; Fournet, R.; Glaude, P.-A.; Sirjean, B. Theoretical Study of the Thermal Decomposition of Urea Derivatives. J. Phys. Chem. A 2022, 126, 6264–6277. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bortoluzzi, M.; Beghetto, V.; Gagliardi, V. Synthesis and Characterization of 6-(1,3-dimethylureido) dibenzo[c,e][1,2]oxaphosphinine 6-oxide. Chem. Proc. 2025, 18, 45. https://doi.org/10.3390/ecsoc-29-26714
Bortoluzzi M, Beghetto V, Gagliardi V. Synthesis and Characterization of 6-(1,3-dimethylureido) dibenzo[c,e][1,2]oxaphosphinine 6-oxide. Chemistry Proceedings. 2025; 18(1):45. https://doi.org/10.3390/ecsoc-29-26714
Chicago/Turabian StyleBortoluzzi, Marco, Valentina Beghetto, and Valeria Gagliardi. 2025. "Synthesis and Characterization of 6-(1,3-dimethylureido) dibenzo[c,e][1,2]oxaphosphinine 6-oxide" Chemistry Proceedings 18, no. 1: 45. https://doi.org/10.3390/ecsoc-29-26714
APA StyleBortoluzzi, M., Beghetto, V., & Gagliardi, V. (2025). Synthesis and Characterization of 6-(1,3-dimethylureido) dibenzo[c,e][1,2]oxaphosphinine 6-oxide. Chemistry Proceedings, 18(1), 45. https://doi.org/10.3390/ecsoc-29-26714






