Preparation, Characterization and in Silico Study of Some Pyrimidine Derivatives That Contain a Chalcone Group and Study of Their Biological Activity †
Abstract
1. Introduction
2. Experimental
2.1. Materials and Methods
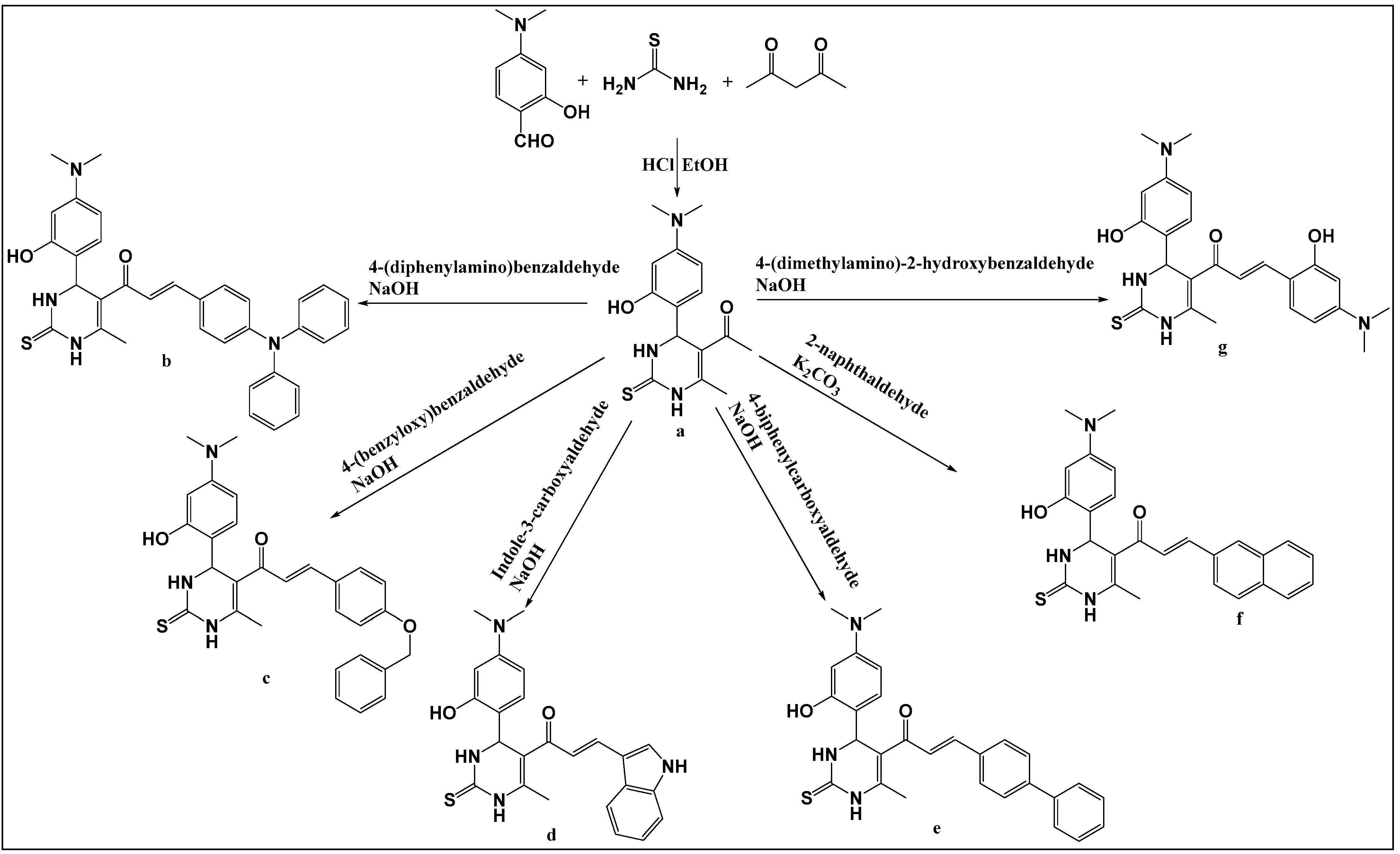
2.2. General Procedure for the Synthesis 1-)4dimethylamino)-2-hydroxyphenyl)-6-methyl-2-thioxo-1,2,3,4-tetrahydropyrimidin-5-yl) ethan-1-one (a)
2.3. General Procedure for the Synthetic Derivatives (b–g)
3. Results and Discussion
3.1. Identification of the Prepared Compounds
3.2. Biological Activity
3.3. In Silico Study
3.3.1. LD50 Test by Protox 3.0 Online
3.3.2. Molecular Docking Study
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shamroukh, A.H.; Rashad, A.E.; Abdelmegeid, F.M.E. The chemistry of pyrido [2,3-d] pyrimidines and their applications. J. Chem. Pharm. Res. 2016, 8, 734–772. [Google Scholar]
- Bhata, A.R.; Dongrea, R.S.; Naikoob, G.A.; Hassanb, I.U.; Araca, T. Proficient synthesis of bioactive annulated pyrimidine de-rivatives. J. Taibah Univ. Sci. 2017, 11, 1047–1069. [Google Scholar] [CrossRef]
- Ashid, M.; Yogi, P.; Katariya, D.; Agarwal, P.; Joshi, A. Pyrmidine: Medical and Biological Significance a Review. World J. Pharm. Pharm. Sci. 2016, 5, 990–1009. [Google Scholar]
- Bansal, S.; Chaudhary, A.N.; Kothiyal, P. Microwave Assisted Synthesis and Antibacterial Activity of Pyrimidine Derivatives. Int. J. Pharm. Pharm. Sci. 2013, 5, 346–348. [Google Scholar]
- Gaonkar, S.L.; Vignesh, U.N. Synthesis and pharmacological properties of chalcones: A review. Res. Chem. Intermed. 2017, 43, 6043–6077. [Google Scholar] [CrossRef]
- Solankee, A.; Tailor, R. An efficient synthesis of some new chalcone, acetyl pyrazoline and amino pyrimidine bearing 1,3,5- triazine nucleus as potential antimicrobial and antitubercular agent. Chem. Int. 2016, 2, 189–200. [Google Scholar]
- Jihad, R.S.; Abdul-Rida, N.A.; Al-Shamari, A.M.J.; Al-Masoudi, N.A.; Saeed, B.A. Design, synthesis, and in-silico study of new letrozole derivatives as prospective anticancer and antioxidant agents. Z. Fur Naturforschung Sect. B-A J. Chem. Sci. 2023, 78, 343–353. [Google Scholar] [CrossRef]
- Mamtora, M.J.; Mahetar, J.G.; Jadeja, J.J.; Manawar, R.B.; Shah, M.K. An efficient Suzuki Reaction Using a New Benzothiazol/pd(II) Species as Catalyst in Aqueous Media. World J. Pharm. Pharm. Sci. 2015, 4, 1046–1052. [Google Scholar]
- Al-Abo, A.M.H. Synthesis of Functionalized Isatins, Benzoxazoles, Isoflavones, Coumarins, by Site-Selective Suzu-ki-Miyaura Cross-Coupling Reactions. Ph.D. Thesis, University Rostock, Rostock, Germany, 2015. [Google Scholar]
- Willemse, T.; Schepens, W.; Van Vlijmen, H.W.T.; Maes, B.U.W.; Ballet, S. Suzuki–Miyaura Cross-Coupling A Versatile Tool. Pept. Diversif. Cyclization Catal. 2017, 7, 74. [Google Scholar] [CrossRef]
- Hela, S.; Younes, M.; Ridha, S. Synthesis of 3,4-dihydropyrimidin-2(1H)-ones/thiones via Biginelli reaction promoted by bismuth(III)nitrate or PPh3 without solvent. Arab. J. Chem. 2016, 9, S510–S514. [Google Scholar]
- Hayam, H.S.; Ahmed, H.S.; Aymn, E.R. Synthesis and biological evaluation of some pyrimidine, pyrimido2,1-b1,3thiazine and thiazolo3,2-apyrimidine derivatives. Acta Pharm. 2006, 56, 231–244. [Google Scholar]
- Silverstein, R.M.; Bassler, G.C. Spectrometric identification of organic compounds. J. Chem. Educ. 1962, 39, 546. [Google Scholar] [CrossRef]
- Hollas, J.M. Modern Spectroscopy, 4th Education; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2004; p. 123. [Google Scholar]
- Sara, S.H. Synthesis and Identifacation of New Type of Antimicrobal Polymer and the Study of Their Biological Activity. Master’s Thesis, Qadisiya University, Al Diwaniyah, Iraq, 2014; pp. 52–54. [Google Scholar]
- Hakan, A.; Nizami, D.; Gulay, B.; Cemal Koray, O.; Cevdet, A. Antimicrobial Activity of Some Thiourea Derivatives and Their Nickel and Copper Complexes. Molecules 2009, 14, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Y.; Zeng, G.; Shao, B.; Chen, M.; Li, Z.; Jiang, Y.; Liu, Y.; Zhang, Y.; Zhong, H. Application of molecular docking for the degradation of organic pollutants in the environmental remediation: A review. Chemosphere 2018, 203, 139–150. [Google Scholar] [CrossRef] [PubMed]

| The Spectrum | The Group | The Derivatives | ||||||
|---|---|---|---|---|---|---|---|---|
| a | b | c | d | e | f | g | ||
| FT-IR (KBr) in cm−1 | N-H&OH | 3391 | 3378 | 3368 | 3380 | 3377 | 3383 | 3403 |
| C-Harom | 3080 | 3108, 3069 | 3008 | 3058 | 3090 | 3090 | 3090 | |
| C-Hali | 2963, 2824 | 2924, 2864 | 2967, 2927 | 2924, 2854 | 2969, 2854 | 2960, 2850 | 2968, 2854 | |
| C=Ocha | - | 1639 | 1654 | 1650 | 1666 | 1650 | 1650 | |
| C=Ccha. | - | 1630 | 1628 | 1630 | 1627 | 1631 | 1630 | |
| C=C arm. | 1597, 1488 | 1594, 1490 | 1554, 1405 | 1584, 1490 | 1566, 1407 | 1560, 1405 | 1569, 1515 | |
| 1H-NMR (DMSO-d6, 400 MHz, δppm) | C19-H | 2.34 | 2.38 | 2.29 | 2.26 | 2.37 | 2.37 | 2.35 |
| C16&17-H | 2.93 | 2.93 | 2.85 | 2.82 | 2.85 | 2.95 | 2.89 | |
| C6-H | 5.82 | 5.84 | 5.83 | 5.90 | 5.85 | 5.77 | 5.78 | |
| C-Har. & C(20+22)-H | 7.29–6.58 | 7.66–6.51 | 7.76–6.34 | 8.29–6.26 | 7.73–6.29 | 8.45–6.20 | 8.41–6.10 | |
| N1-H | 9.39 | 9.34 | 9.81 | 9.04 | 9.57 | 9.23 | 9.10 | |
| N3-H | 9.72 | 9.78 | 9.88 | 9.12 | 9.64 | 9.68 | 9.60 | |
| O-H | 10.81 | 10.26 | 10.67 | 11.09 | 10.14 | 10.00 | 10.65 | |
| others | 2.25 (C20-H) | - | 5.01 (C30-H) | - | - | - | 8.82 (C32-OH) | |
| 13C-NMR (DMSO-d6, 100 MHz, δppm) | C-19 | 19.03 | 19.03 | 19.01 | 19.68 | 19.35 | 19.43 | 19.01 |
| C-(16+17) | 39.36 | 40.63 | 39.83 | 39.67 | 39.56 | 39.91 | 39.84 | |
| C-6 | 51.35 | 51.49 | 51.81 | 51.84 | 50.81 | 49.29 | 50.25 | |
| C-5 | 112.91 | 112.69 | 112.91 | 112.63 | 113.40 | 113.60 | 112.35 | |
| C-20 | 30.01 | 127.13 | 127.60 | 127.62 | 127.58 | 127.45 | 126.81 | |
| C-22 | - | 147.28 | 145.33 | 136.36 | 145.68 | 145.34 | 144.99 | |
| C-4 | 151.34 | 148.62 | 148.46 | 149.16 | 149.86 | 148.53 | 148.53 | |
| Car. | 157.11–99.62 | 159.68–99.83 | 158.48–100.47 | 155.28–98.56 | 155.52–99.55 | 155.96–99.20 | 157.23–97.88 | |
| C-2 | 173.33 | 176.52 | 175.87 | 173.98 | 174.21 | 175.32 | 174.17 | |
| C-18 | 189.44 | 185.32 | 185.41 | 185.69 | 185.71 | 186.37 | 184.59 | |
| others | - | - | 68.73 (C-30) | - | - | - | 39.65 C-(30 + 31) 97.88 (C-30) | |
| No. | Formula | m.p (oC) | Color | Rf | Yield (%) | Phase |
|---|---|---|---|---|---|---|
| a | 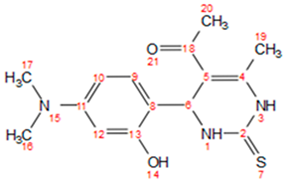 | 190–191 | Brown | 0.54 | 89 | solid |
| b |  | 193–194 | Brown | 0.58 | 77 | solid |
| c | 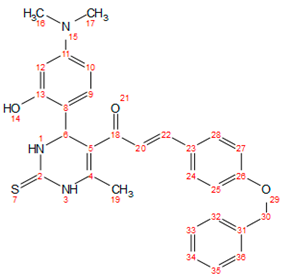 | 198–200 | Blackish brown | 0.61 | 79 | solid |
| d | 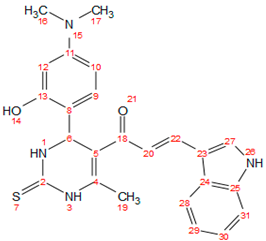 | 187–189 | Red | 0.67 | 82 | solid |
| e | 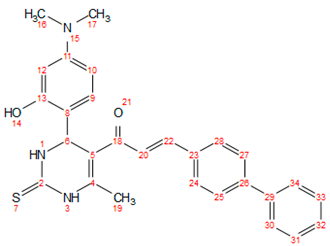 | 178–180 | Brown | 0.63 | 75 | solid |
| f | 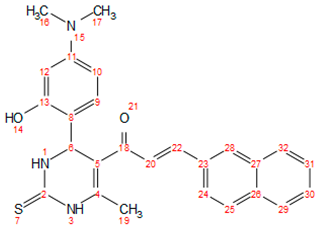 | 165–167 | Blackish brown | 0.77 | 85 | solid |
| g |  | 173–175 | Red | 0.69 | 81 | solid |
| no. | a | b | c | d | e | f | g |
|---|---|---|---|---|---|---|---|
| Inhibition zones (mm) | 2 | 3 | 1 | 14 | 2 | 11 | 15 |
| The Derivative | a | b | c | d | e | f | g |
|---|---|---|---|---|---|---|---|
| LD50 (mg/Kg) | 1700 | 1700 | 785 | 785 | 1700 | 1700 | 1700 |
| The Derivative | Docking Score (kcal/mol) | Ranking | rsmd | Amino Acid Interaction |
|---|---|---|---|---|
| a | −7.2071 | 1 | 1.4506 | SER 314, VAL 369, VAL 370 |
| b | −9.5734 | 1 | 1.5638 | MET 374 |
| c | −10.1140 | 1 | 1.8279 | ARG 115, ARG 375 |
| d | −8.7276 | 2 | 1.0964 | ARG 115, ARG 375, GLY 431, GLY 436, THR 310 |
| e | −8.9375 | 1 | 1.7236 | ARG 115, ARG 375 |
| f | −8.4432 | 1 | 1.7314 | ALA 438, ARG 115, THR 141, ARG 435, PHE 430 |
| g | −9.1268 | 1 | 1.4235 | ARG 115, ARG 375 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulameer, S.R.; Jihad, R.S.; Alghanmy, H.S. Preparation, Characterization and in Silico Study of Some Pyrimidine Derivatives That Contain a Chalcone Group and Study of Their Biological Activity. Chem. Proc. 2025, 18, 42. https://doi.org/10.3390/ecsoc-29-26827
Abdulameer SR, Jihad RS, Alghanmy HS. Preparation, Characterization and in Silico Study of Some Pyrimidine Derivatives That Contain a Chalcone Group and Study of Their Biological Activity. Chemistry Proceedings. 2025; 18(1):42. https://doi.org/10.3390/ecsoc-29-26827
Chicago/Turabian StyleAbdulameer, Salwa R., Raad Saad Jihad, and Hiba Salman Alghanmy. 2025. "Preparation, Characterization and in Silico Study of Some Pyrimidine Derivatives That Contain a Chalcone Group and Study of Their Biological Activity" Chemistry Proceedings 18, no. 1: 42. https://doi.org/10.3390/ecsoc-29-26827
APA StyleAbdulameer, S. R., Jihad, R. S., & Alghanmy, H. S. (2025). Preparation, Characterization and in Silico Study of Some Pyrimidine Derivatives That Contain a Chalcone Group and Study of Their Biological Activity. Chemistry Proceedings, 18(1), 42. https://doi.org/10.3390/ecsoc-29-26827





