Synthesis of Biologically Active Arginine Derivatives Derived from Salicylamide †
Abstract
1. Introduction
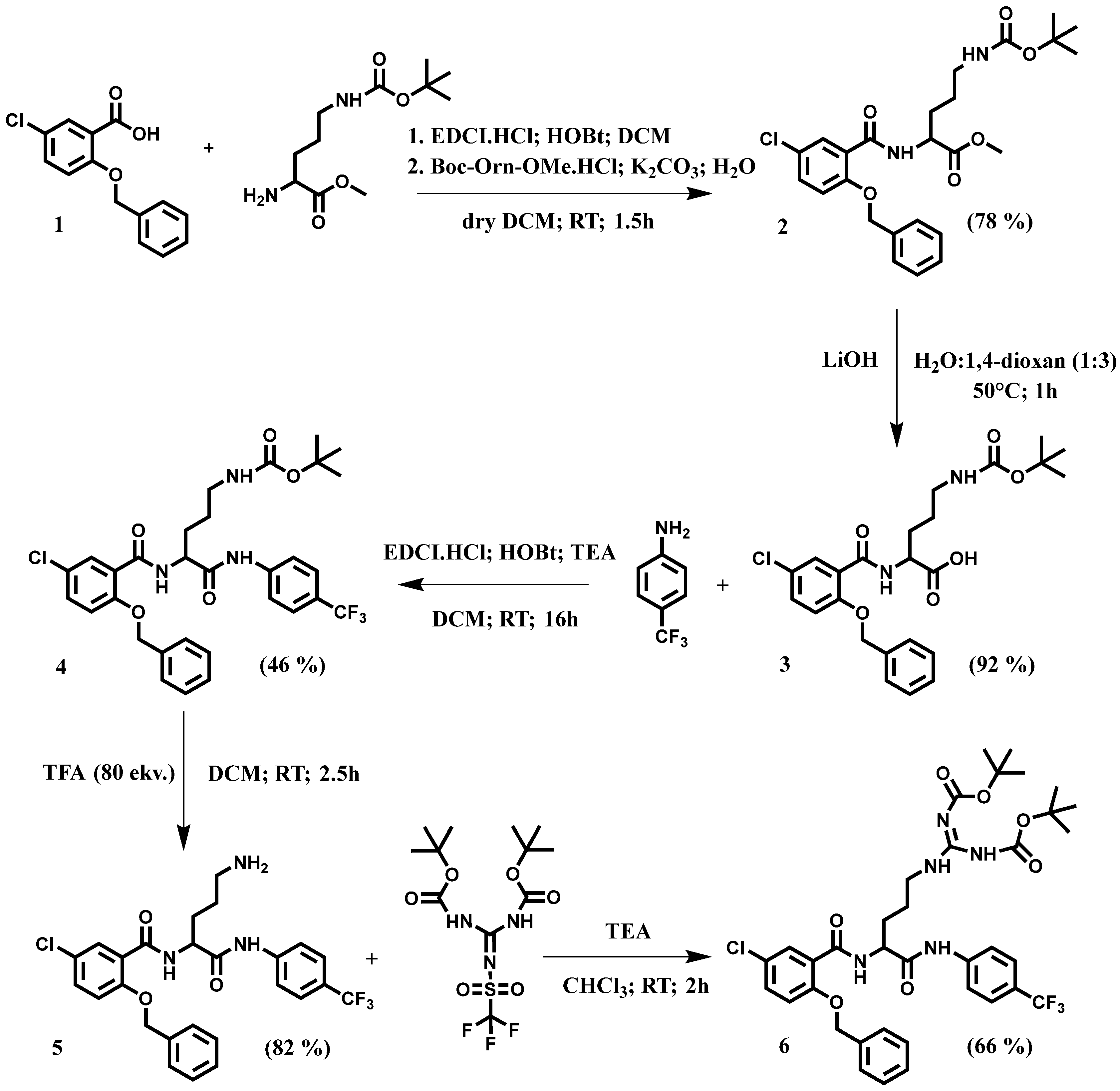
2. Results and Discussion
3. Conclusions
4. Material and Methods
4.1. Chemistry
4.2. Experimental Procedures for Synthesis of Intermediates, Key Ornithine Intermediate and Final Compound
4.2.1. Experimental Procedure for Synthesis of Methylester 2
4.2.2. Experimental Procedure for Synthesis of Carboxylic Acid 3
4.2.3. Experimental Procedure for Synthesis of Peptidomimetics with Anilid Part 4
4.2.4. Experimental Procedure for Synthesis of Key Ornithine Intermediate 5
4.2.5. Experimental Procedure for Synthesis of Final Arginine Peptidomimetic 6
4.3. Characterization of Intermediates, Key Ornithine Intermediate and Final Compound
4.3.1. Characterization of Methylester 2
4.3.2. Characterization of Carboxylic Acid 3
4.3.3. Characterization of Peptidomimetic with Anilid Part 4
4.3.4. Characterization of Key Ornithine Intermediate 5
4.3.5. Characterization of Final Arginine Peptidomimetic 6
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| EDCI·HCl | N-ethyl-N′-(3-dimethylaminopropyl)carbodiimide hydrochloride |
| EtOAc | Ethyl acetate |
| HOBt | 1-hydroxybenzotriazole |
| HRMS | High-resolution mass spectrometry |
| mp | Melting point |
| NMR | Nuclear magnetic resonance |
| TEA | Triethylamine |
| TFA | Trifluoroacetic acid |
| TLC | Thin-layer chromatography |
References
- Lee, Y.S. Peptidomimetics and Their Applications for Opioid Peptide Drug Discovery. Biomolecules 2022, 12, 1241. [Google Scholar] [CrossRef] [PubMed]
- Adang, A.E.P.; Hermkens, P.H.H.; Linders, J.T.M.; Ottenheijm, H.C.J.; Van Staveren, C.J. Case Histories of Peptidomimetics: Progression from Peptides to Drugs. Recueil des Travaux Chimiques des Pays-Bas 1994, 113, 63–78. [Google Scholar] [CrossRef]
- Ding, D.; Xu, S.; da Silva-Júnior, E.F.; Liu, X.; Zhan, P. Medicinal Chemistry Insights into Antiviral Peptidomimetics. Drug Discov. Today 2023, 28, 103468. [Google Scholar] [CrossRef] [PubMed]
- Jorda, R.; Dušek, J.; Řezníčková, E.; Pauk, K.; Magar, P.P.; Imramovský, A.; Kryštof, V. Synthesis and Antiproteasomal Activity of Novel O-Benzyl Salicylamide-Based Inhibitors Built from Leucine and Phenylalanine. Eur. J. Med. Chem. 2017, 135, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Jorda, R.; Magar, P.; Hendrychová, D.; Pauk, K.; Dibuš, M.; Pilařová, E.; Imramovský, A.; Kryštof, V. Novel Modified Leucine and Phenylalanine Dipeptides Modulate Viability and Attachment of Cancer Cells. Eur. J. Med. Chem. 2020, 188, 112036. [Google Scholar] [CrossRef] [PubMed]
- Jorda, R.; Molitorová, V.; Pilařová, E.; Vojáčková, V.; Řezníčková, E.; Svobodová, K.; Pauk, K.; Imramovský, A.; Kryštof, V. Pseudopeptides with Aldehyde or Vinylsulfone Warheads: Synthesis and Antiproteasomal Activity. Bioorg. Chem. 2021, 115, 105228. [Google Scholar] [CrossRef] [PubMed]
- Baldassarre, L.; Pinnen, F.; Cornacchia, C.; Fornasari, E.; Cellini, L.; Baffoni, M.; Cacciatore, I. Synthesis of Short Cationic Antimicrobial Peptidomimetics Containing Arginine Analogues. J. Pept. Sci. 2012, 18, 567–578. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, C.; Zhang, L.; Weigel, L.F.; Schilz, J.; Graf, D.; Bartenschlager, R.; Hilgenfeld, R.; Klein, C.D. Peptide-Boronic Acid Inhibitors of Flaviviral Proteases: Medicinal Chemistry and Structural. Biol. J. Med. Chem. 2017, 60, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Mahindra, A.; Bagra, N.; Wangoo, N.; Khan, S.I.; Jacob, M.R.; Jain, R. Discovery of Short Peptides Exhibiting High Potency against Cryptococcus Neoformans. ACS Med. Chem. Lett. 2014, 5, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Colombo, R.; Mingozzi, M.; Belvisi, L.; Arosio, D.; Piarulli, U.; Carenini, N.; Perego, P.; Zaffaroni, N.; De Cesare, M.; Castiglioni, V.; et al. Synthesis and Biological Evaluation (in Vitro and in Vivo) of Cyclic Arginine-Glycine-Aspartate (RGD) Peptidomimetic-Paclitaxel Conjugates Targeting Integrin Avβ3. J. Med. Chem. 2012, 55, 10460–10474. [Google Scholar] [CrossRef] [PubMed]
- Fareed, J.; Jeske, W.P. Small-Molecule Direct Antithrombins: Argatroban. Best Pract. Res. Clin. Haematol. 2004, 17, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, T.; Kamijo, S. Inhibition of Aggregation of Amyloid Β42 by Arginine-Containing Small Compounds. Biosci. Biotechnol. Biochem. 2012, 76, 762–766. [Google Scholar] [CrossRef] [PubMed]
- Bihel, F.; Humbert, J.P.; Schneider, S.; Bertin, I.; Wagner, P.; Schmitt, M.; Laboureyras, E.; Petit-Demouliere, B.; Schneider, E.; Mollereau, C.; et al. Development of a Peptidomimetic Antagonist of Neuropeptide FF Receptors for the Prevention of Opioid-Induced Hyperalgesia. ACS Chem. Neurosci. 2015, 6, 438–445. [Google Scholar] [CrossRef] [PubMed]
- El-Faham, A.; Albericio, F. Peptide Coupling Reagents, More than a Letter Soup. Chem. Rev. 2011, 111, 6557–6602. [Google Scholar] [CrossRef] [PubMed]
- Peterlin-Mašić, L.; Kikelj, D. Arginine Mimetics. Tetrahedron 2001, 57, 7073–7105. [Google Scholar] [CrossRef]
- Gaudioso, L.A.; Weglarz, M. Novel Process for the Synthesis of Exochelins. WO 00/09547 A1, 24 February 2000. World Intellectual Property Organization. pp. 21–22. [Google Scholar]
- Soengas, R.G.; Larrosa, M.; Balado, M.; Rodríguez, J.; Lemos, M.L.; Jiménez, C. Synthesis and Biological Activity of Analogues of Vanchrobactin, a Siderophore from Vibrio Anguillarum Serotype O2. Org. Biomol. Chem. 2008, 6, 1278–1287. [Google Scholar] [CrossRef] [PubMed]
- Fulmer, G.R.; Miller, A.J.M.; Sherden, N.H.; Gottlieb, H.E.; Nudelman, A.; Stoltz, B.M.; Bercaw, J.E.; Goldberg, K.I. NMR Chemical Shifts of Trace Impurities: Common Laboratory Solvents, Organics, and Gases in Deuterated Solvents Relevant to the Organometallic Chemist. Organometallics 2010, 29, 2176–2179. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šanda, M.; Imramovský, A. Synthesis of Biologically Active Arginine Derivatives Derived from Salicylamide. Chem. Proc. 2025, 18, 32. https://doi.org/10.3390/ecsoc-29-26862
Šanda M, Imramovský A. Synthesis of Biologically Active Arginine Derivatives Derived from Salicylamide. Chemistry Proceedings. 2025; 18(1):32. https://doi.org/10.3390/ecsoc-29-26862
Chicago/Turabian StyleŠanda, Martin, and Aleš Imramovský. 2025. "Synthesis of Biologically Active Arginine Derivatives Derived from Salicylamide" Chemistry Proceedings 18, no. 1: 32. https://doi.org/10.3390/ecsoc-29-26862
APA StyleŠanda, M., & Imramovský, A. (2025). Synthesis of Biologically Active Arginine Derivatives Derived from Salicylamide. Chemistry Proceedings, 18(1), 32. https://doi.org/10.3390/ecsoc-29-26862





