One-Pot Synthesis of 3-Tetrazolylmethyl-4H-Chromen-4-Ones via Ugi-Azide Reaction †
Abstract
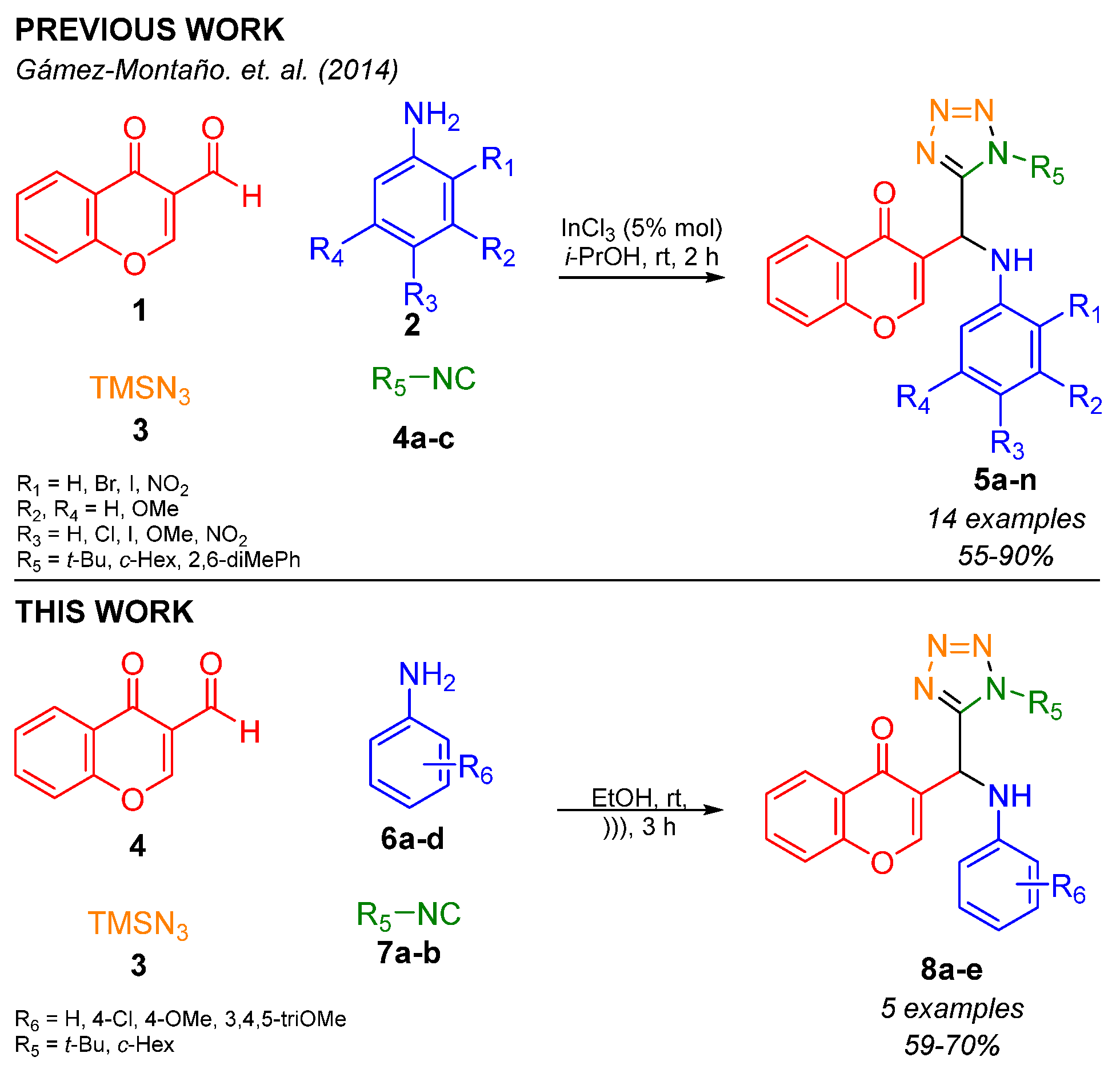
1. Introduction
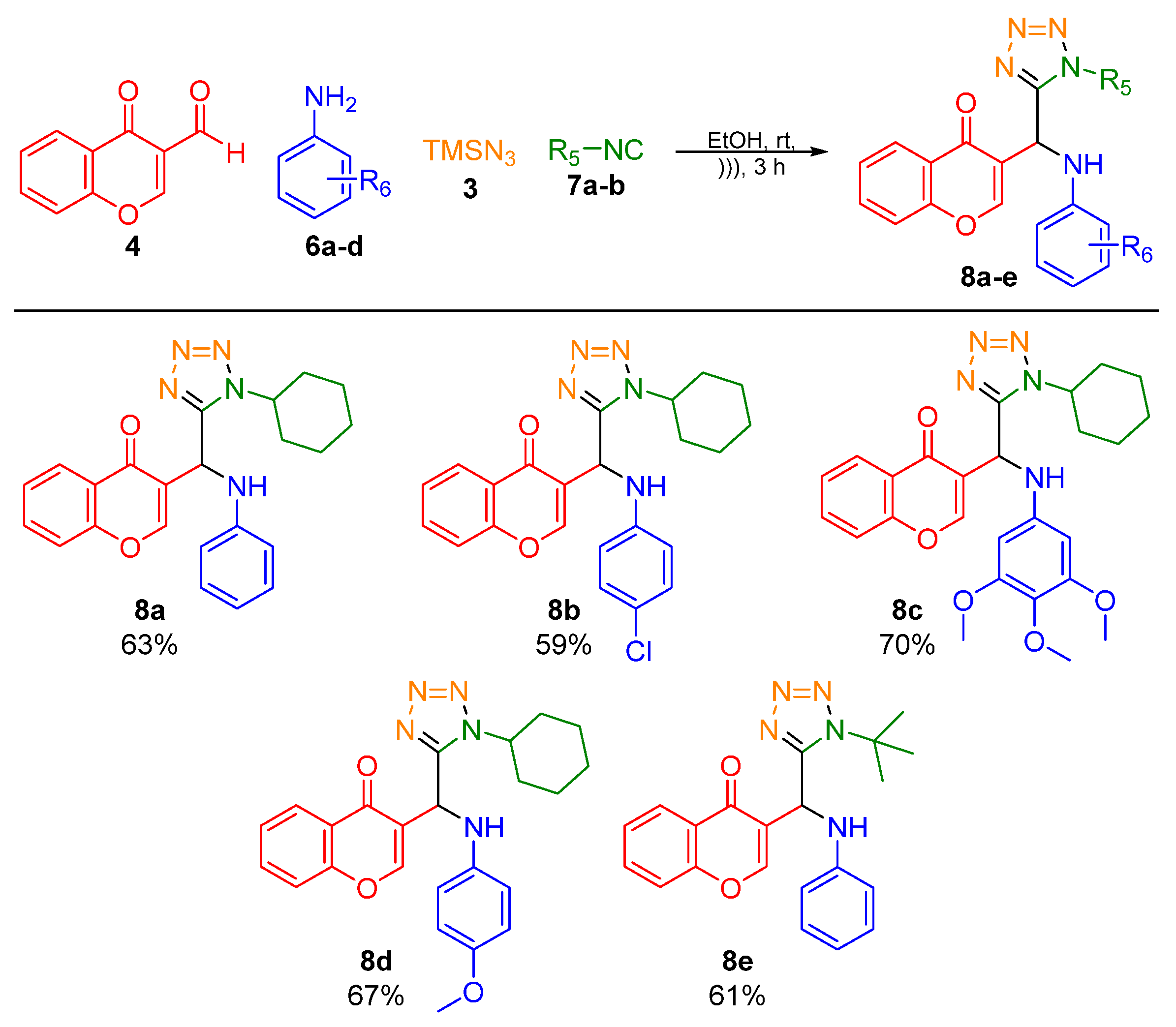
2. Results and Discussion
3. Experimental Methods
3.1. General Experimental Information
3.2. Procedure
3.3. Spectral Data
- 3-((1-cyclohexyl-1H-tetrazol-5-yl)(phenylamino)methyl)-4H-chromen-4-one (8a)
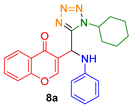
- 3-(((4-chlorophenyl)amino)(1-cyclohexyl-1H-tetrazol-5-yl)methyl)-4H-chromen-4-one (8b)
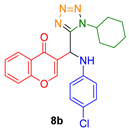
- 3-((1-cyclohexyl-1H-tetrazol-5-yl)((3,4,5-trimethoxyphenyl)amino)methyl)-4H-chromen-4-one (8c)
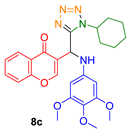
- 3-((1-cyclohexyl-1H-tetrazol-5-yl)((4-methoxyphenyl)amino)methyl)-4H-chromen-4-one (8d)

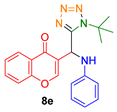
- 3-((1-(tert-butyl)-1H-tetrazol-5-yl)(phenylamino)methyl)-4H-chromen-4-one (8e)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buntrock, R.E. Review of Heterocyclic Chemistry, 5th Edition. J. Chem. Educ. 2012, 89, 1349–1350. [Google Scholar] [CrossRef]
- Marshall, C.M.; Federice, J.G.; Bell, C.N.; Cox, P.B.; Njardarson, J.T. An Update on the Nitrogen Heterocycle Compositions and Properties of U.S. FDA-Approved Pharmaceuticals (2013–2023). J. Med. Chem. 2024, 67, 11622–11655. [Google Scholar] [CrossRef] [PubMed]
- Al-Jumaili, M.H.A.; Hamad, A.A.; Hashem, H.E.; Hussein, A.D.; Muhaidi, M.J.; Ahmed, M.A.; Albanaa, A.H.A.; Siddique, F.; Bakr, E.A. Comprehensive Review on the Bis–Heterocyclic Compounds and Their Anticancer Efficacy. J. Mol. Struct. 2023, 1271, 133970. [Google Scholar] [CrossRef]
- Zhu, J.; Bienaymé, H. (Eds.) Front Matter. In Multicomponent Reactions; Wiley: Hoboken, NJ, USA, 2005. [Google Scholar] [CrossRef]
- Zhu, J. Multicomponent Reactions in Organic Synthesis; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Hayashi, Y. Pot Economy and One-Pot Synthesis. Chem. Sci. 2016, 7, 866–880. [Google Scholar] [CrossRef] [PubMed]
- Neochoritis, C.G.; Zhao, T.; Dömling, A. Tetrazoles via Multicomponent Reactions. Chem. Rev. 2019, 119, 1970–2042. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Wang, Y.; Yan, S.; Zhang, Y.; Ma, X.; Zhang, Q.; Zhang, W. One-Pot Ugi-Azide and Heck Reactions for the Synthesis of Heterocyclic Systems Containing Tetrazole and 1,2,3,4-Tetrahydroisoquinoline. Beilstein J. Org. Chem. 2024, 20, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Pharande, S.G.; Corrales Escobosa, A.R.; Gámez-Montaño, R. Endogenous Water-Triggered and Ultrasound Accelerated Synthesis of 1,5-Disubstituted Tetrazoles via a Solvent and Catalyst-Free Ugi-Azide Reaction. Green Chem. 2017, 19, 1259–1262. [Google Scholar] [CrossRef]
- Pharande, S.G.; Rentería-Gómez, M.A.; Gámez-Montaño, R. Isocyanide Based Multicomponent Click Reactions: A Green and Improved Synthesis of 1-Substituted 1 H-1,2,3,4-Tetrazoles. New J. Chem. 2018, 42, 11294–11298. [Google Scholar] [CrossRef]
- Swami, S.; Shrivastava, R.; Sharma, N.; Agarwala, A.; Verma, V.P.; Singh, A.P. An Ultrasound-Assisted Solvent and Catalyst-Free Synthesis of Structurally Diverse Pyrazole Centered 1,5-Disubstituted Tetrazoles via One-Pot Four-Component Reaction. Lett. Org. Chem. 2022, 19, 795–802. [Google Scholar] [CrossRef]
- Unnamatla, M.V.B.; Islas-Jácome, A.; Quezada-Soto, A.; Ramírez-López, S.C.; Flores-Álamo, M.; Gámez-Montaño, R. Multicomponent One-Pot Synthesis of 3-Tetrazolyl and 3-Imidazo[1,2-a]Pyridin Tetrazolo[1,5-a]Quinolines. J. Org. Chem. 2016, 81, 10576–10583. [Google Scholar] [CrossRef] [PubMed]
- Rentería-Gómez, A.; Islas-Jácome, A.; Cruz-Jiménez, A.E.; Manzano-Velázquez, J.C.; Rojas-Lima, S.; Jiménez-Halla, J.O.C.; Gámez-Montaño, R. Synthesis of 2-Tetrazolylmethyl-Isoindolin-1-Ones via a One-Pot Ugi-Azide/(N-Acylation/Exo-Diels–Alder)/Dehydration Process. ACS Omega 2016, 1, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Vicente–García, E.; Kielland, N.; Lavilla, R. Functionalization of Heterocycles by MCRs. In Multicomponent Reactions in Organic Synthesis; Zhu, J., Wang, Q., Wang, M., Eds.; Wiley: Hoboken, NJ, USA, 2014; pp. 159–182. [Google Scholar] [CrossRef]
- Cano, P.A.; Islas-Jácome, A.; González-Marrero, J.; Yépez-Mulia, L.; Calzada, F.; Gámez-Montaño, R. Synthesis of 3-Tetrazolylmethyl-4H-Chromen-4-Ones via Ugi-Azide and Biological Evaluation against Entamoeba Histolytica, Giardia Lamblia and Trichomona Vaginalis. Bioorg. Med. Chem. 2014, 22, 1370–1376. [Google Scholar] [CrossRef] [PubMed]
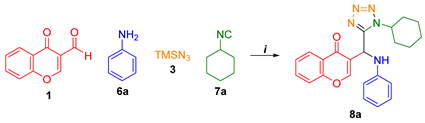 | ||||
|---|---|---|---|---|
| Entry | Solvent | Temperature | Time | Yield (%) |
| 1 | EtOH a | r.t. | 12 h | 56 |
| 2 | --- b | r.t. | 3 h | Traces |
| 3 | EtOH b | r.t. | 3 h | 63 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-García, D.; González-Pérez, K.A.; González-Gámez, I.A.; Gámez-Montaño, R. One-Pot Synthesis of 3-Tetrazolylmethyl-4H-Chromen-4-Ones via Ugi-Azide Reaction. Chem. Proc. 2025, 18, 23. https://doi.org/10.3390/ecsoc-29-26855
García-García D, González-Pérez KA, González-Gámez IA, Gámez-Montaño R. One-Pot Synthesis of 3-Tetrazolylmethyl-4H-Chromen-4-Ones via Ugi-Azide Reaction. Chemistry Proceedings. 2025; 18(1):23. https://doi.org/10.3390/ecsoc-29-26855
Chicago/Turabian StyleGarcía-García, Diana, Karla A. González-Pérez, Indhira A. González-Gámez, and Rocío Gámez-Montaño. 2025. "One-Pot Synthesis of 3-Tetrazolylmethyl-4H-Chromen-4-Ones via Ugi-Azide Reaction" Chemistry Proceedings 18, no. 1: 23. https://doi.org/10.3390/ecsoc-29-26855
APA StyleGarcía-García, D., González-Pérez, K. A., González-Gámez, I. A., & Gámez-Montaño, R. (2025). One-Pot Synthesis of 3-Tetrazolylmethyl-4H-Chromen-4-Ones via Ugi-Azide Reaction. Chemistry Proceedings, 18(1), 23. https://doi.org/10.3390/ecsoc-29-26855






