Simple Synthesis of New Bioactive Nitrogenous Compounds by In Silico Study †
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Used
2.2. Methods
2.2.1. Ligand Preparation
2.2.2. Targets Preparation:
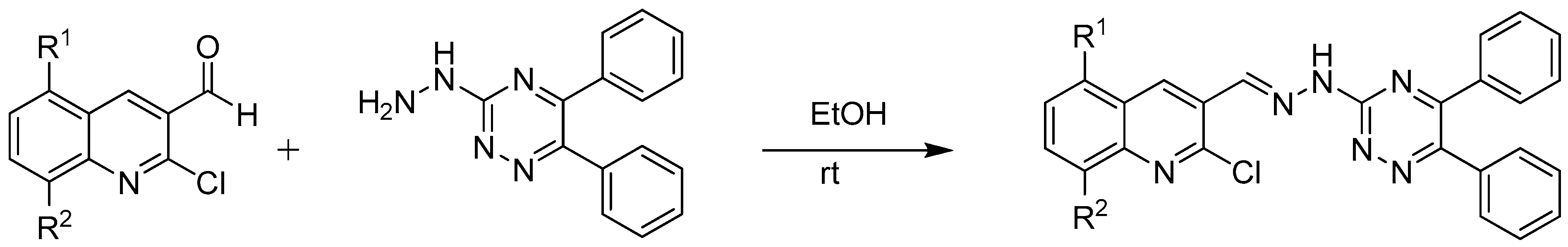
2.2.3. Synthesis of Compounds:
3. Results and Discussion
3.1. Docking Study
3.2. Pharmacokinetic Study
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, H.; Dhameja, M.; Kurella, S.; Uma, A.; Gupta, P. Synthesis, in-vitro α-glucosidase inhibition and molecular docking studies of 1,3,4-thiadiazole-5,6-diphenyl-1,2,4-triazine hybrids: Potential leads in the search of new antidiabetic drugs. J. Mol. Struct. 2023, 1273, 134339. [Google Scholar] [CrossRef]
- Ge, X.; Xu, Z. 1,2,4-Triazole hybrids with potential antibacterial activity against methicillin-resistant Staphylococcus aureus. Arch. Pharm. 2020, 354, 2000223. [Google Scholar] [CrossRef] [PubMed]
- Yazdani, M.; Edraki, N.; Badri, R.; Khoshneviszadeh, M.; Iraji, A.; Firuzi, O. Multi-target inhibitors against Alzheimer disease derived from 3-hydrazinyl 1,2,4-triazine scaffold containing pendant phenoxy methyl-1,2,3-triazole: Design, synthesis and biological evaluation. Bioorg. Chem. 2019, 84, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Gornowicz, A.; Szymanowska, A.; Mojzych, M.; Czarnomysy, R.; Bielawski, K.; Bielawska, A. The Anticancer Action of a Novel 1,2,4-Triazine Sulfonamide Derivative in Colon Cancer Cells. Molecules 2021, 26, 2045. [Google Scholar] [CrossRef] [PubMed]
- Mokrani, E.H.; Bensegueni, A.; Chaput, L.; Beauvineau, C.; Djeghim, H.; Mouawad, L. Identification of New Potent Acetylcholinesterase Inhibitors Using Virtual Screening and In Vitro Approaches. Mol. Inform. 2019, 38, 1800118. [Google Scholar] [CrossRef] [PubMed]
- Huzil, J.T.; Ludueña, R.F.; Tuszynski, J. Comparative modelling of human β tubulin isotypes and implications for drug binding. Nanotechnology 2006, 17, S90–S100. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.S.; Ge, Y.X.; Cheng, Z.Q.; Song, J.L.; Wang, Y.Y.; Zhu, K.; Zhang, H. Discovery of new multifunctional selective acetylcholinesterase inhibitors: Structure based virtual screening and biological evaluation. J. Comput. Aided. Mol. Des. 2019, 33, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Sana, S.; Reddy, V.G.; Reddy, T.S.; Tokala, R.; Kumar, R.; Bhargava, S.K.; Shankaraiah, N. Cinnamide derived pyrimidine-benzimidazole hybrids as tubulin inhibitors: Synthesis, in silico and cell growth inhibition studies. Bioorg. Chem. 2021, 110, 104765. [Google Scholar] [CrossRef] [PubMed]





| Entries | Lipinski’s Rules | Veber’s Rules | BBB | AMES Toxic | Carcinogenic | P-glycoprotein Inhibitor |
|---|---|---|---|---|---|---|
| A 1 | + | + | + | - | - | - |
| A 2 | + | + | + | - | - | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ait Belkacem, A. Simple Synthesis of New Bioactive Nitrogenous Compounds by In Silico Study. Chem. Proc. 2024, 16, 106. https://doi.org/10.3390/ecsoc-28-20251
Ait Belkacem A. Simple Synthesis of New Bioactive Nitrogenous Compounds by In Silico Study. Chemistry Proceedings. 2024; 16(1):106. https://doi.org/10.3390/ecsoc-28-20251
Chicago/Turabian StyleAit Belkacem, Amira. 2024. "Simple Synthesis of New Bioactive Nitrogenous Compounds by In Silico Study" Chemistry Proceedings 16, no. 1: 106. https://doi.org/10.3390/ecsoc-28-20251
APA StyleAit Belkacem, A. (2024). Simple Synthesis of New Bioactive Nitrogenous Compounds by In Silico Study. Chemistry Proceedings, 16(1), 106. https://doi.org/10.3390/ecsoc-28-20251





