Abstract
New hydrazinecarboximidamide derivatives were successfully synthesized with excellent yields. These compounds have been proven to be potent inhibitors of acetylcholinesterase and tubulin, and they also exhibit significant antioxidant effects. Furthermore, an ADMET study was conducted, which suggests that these compounds not only possess the potential to cross the blood-brain barrier. These findings allow us to consider this product as a promising candidate for future medicinal development, particularly in the treatment of neurodegenerative diseases and cancer.
1. Introduction
Hydrazine compounds have wide applications and are very interesting, including a wide range of biological activities, antibacterial [1], antidiabetic [2], antiviral [3], antimicrobial [4], anticancer [5], Anti-Alzheimer [6] and antioxidant [7]. Such properties make these compounds particularly promising for the development of treatments targeting oxidative stress-related diseases.
The presence of numerous nitrogen atoms in these compounds grants them strong chelation properties with free radicals, which in turn significantly enhances their ability to interact effectively with target molecules. Such properties make these compounds particularly promising for the development of treatments targeting oxidative stress-related diseases.
New hydrazine derivatives were synthesized using a simple condensation reaction. These compounds have shown strong inhibitory effects on acetylcholinesterase and tubulin, as well as antioxidant properties. Furthermore, ADMET analysis confirms that these compounds adhere to Lipinski’s Rule of Five and can cross the blood-brain barrier without exhibiting any ADMET-related toxicity.
2. Materials and Methods
2.1. Material Used
The ligand was prepared using the Avogadro program, while target preparation was carried out in Chimera version 1.16. Energy minimization was performed using Swiss-PdbViewer (SPDBV) version 4.1, and the visualization and analysis were conducted using BIOVIA Discovery Studio 2021, DPPH analyse using ANOVA SPSS software 2.0.
2.2. Statistical Analysis
The reported results represent the average of three measurements, expressed as mean ± standard deviation (SD). The IC50 value, indicating the concentration required for 50% inhibition, was determined for each sample by plotting the percentage of inhibition against concentration and analyzing the resulting curve using linear regression. To identify statistically significant differences (p < 0.05) between the samples, a one-way analysis of variance (ANOVA) was performed using SPSS software.
2.3. Methods
2.3.1. Ligand Preparation
Preparation of derivative compounds for docking studies involved to designing small molecules, optimize their geometry, and minimize their energy. The resulting structures were then saved in the .pdb format.
2.3.2. Targets Preparation
The acetylcholinesterase (AChE) protein (PDB ID: 4m0e) [8], and tubulin protein (PDB ID: 1sa0) [9], were obtained in .pdb format from the Protein Data Bank. The targets were prepared by removing water molecules, trimming unnecessary chains, and adding polar hydrogens. Energy minimization was then performed, and the results were visualized and analyzed.
2.3.3. Synthesis of Compounds
An equimolar mixture of quinoline derivatives and aminoguanidine was stirred at reflex in methanol (MeOH) without a catalyst. The precipitate was then filtered, washed with MeOH, and recrystallized from MeOH.
3. Results and Discussion
Hydrazine carboximidamides are easily synthesized through a simple condensation reaction (Scheme 1) between quinoline carbaldehyde and aminoguanidine, without the use of a catalyst. The products obtained are (E)-2-((2-chloro-5,8-dimethoxyquinolin-3-yl)methylene)hydrazinecarboximidamide and (E)-2-((2-chloro-6-methoxyquinolin-3-yl)methylene)hydrazinecarboximidamide, with yields ranging from 93% to 98%.These compounds are identified by 1H NMR, The characteristic CHN condensation peak appears at 8.75 ppm.
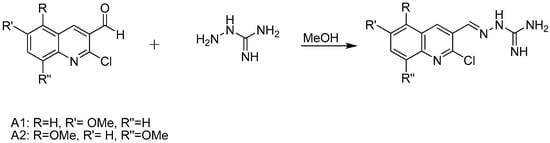
Scheme 1.
General procedure for synthesis.
3.1. Docking Study
The docking study of these compounds was performed using Chimera to evaluate their inhibitory ability. In this study, the active site of the target atoms was fixed, while the chromophore remained flexible. As is well known, this program uses a genetic algorithm (GA) to dock flexible ligands into the protein binding site.
The molecular docking study indicated a strong affinity and high scores within the active sites of tubulin, suggesting potential anticancer activity. However, docking with acetylcholinesterase showed a high score but with unfavorable binding, highlighting the compounds’ potential for anticancer and anti-Alzheimer’s activities (Figure 1, Figure 2, Figure 3 and Figure 4).
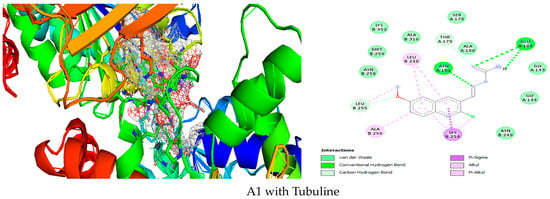
Figure 1.
Simulation of A1 in the active site of Tubuline.
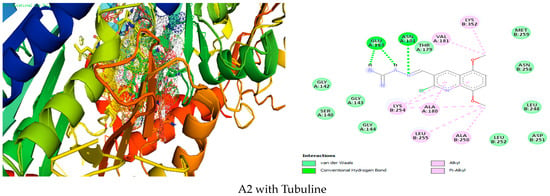
Figure 2.
Simulation of A2 in the active site of Tubuline.
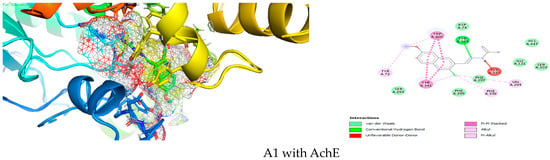
Figure 3.
Simulation of A1 in the active site of AChE.
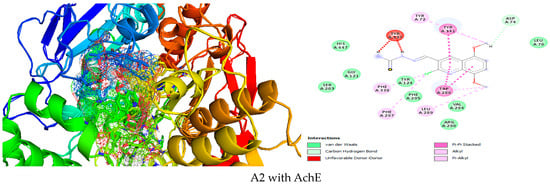
Figure 4.
Simulation of A2 in the active site of AChE.
As we can see A1 is inside Tubulin (Figure 1), with good affinity due to Van der Waals interactions with ASN B:258, MET B:259, LYS B:352, ALA B:316, SER A:178, ALA A:180, GLY A:143, GLY A:144, ASN B:249, hydrogen bond GLU A:183, ASN A:101, carbon hydrogen bond THR A:179, Pi-Sigma LYS B:254,Pi- alkyl, alkyl ALA B:250, LEU B:248.
As we can see in (Figure 2), A2 is inside Tubulin with good affinity due to Van der Waals interactions with THR A:179, MET B:259, ASN B:258, LEU B:248, ASP B:251, LEU B:252, GLY A:144, SER A:140, GLY A:143, GLY A:142, hydrogen bond GLU A:183, ASN A:101, Alkyl and Pi Alkyl VAL A:181, LYS B:352, ALA B:250, LEU B:255, ALA A:180, LYS B:254.
A1 is inside AchE (Figure 3), with good affinity due to Van der Waals interactions with ASP A:74, HIS A:447, GLY A:121, SER A:203, PHE A:297, PHE A:295, SER A:293, hydrogen bond TYR A:124, Pi-Pi stacked tyr A:341, trp A:286, VAL A:294, PHE A:338, TYR A:72.
A2 is inside AchE (Figure 4), with good affinity due to Van der Waals interactions with HIS A:447, GLY A:121, SER A:203, TYR A:124, PHE A:295, ARG A:296, VAL A:294, LEU A:76, carbon hydrogen asp A:74,Pi-Pi stacked TYR A:341, TRP A:286, Alkyl TYR A:72, LEU A:289, PHE A:297, PHE A:338, unfavorable donor-donor TYR A:337.
3.2. Pharmacokinetic Study
According to the ADMET study by SwissADME (http://www.swissadme.ch/index.php, accessed on 6 August 2024), both A1 and A2 adhere to Lipinski’s rules and Veber’s rules. By ADMETSAR, both compounds are able to cross the blood-brain barrier, which suggests they may reach the brain and potentially be effective as anti-Alzheimer’s agents. Additionally, we used admetSAR (http://lmmd.ecust.edu.cn/admetsar1, accessed on 6 August 2024) to confirm that they are non-AMES toxic and non-carcinogenic (Table 1).

Table 1.
Table of ADMET study results.
3.3. Antioxidant Evaluation
Estimation of DPPH Free Radical Scavenging Activity
The antioxidant activity of two synthesized products, was evaluated using a modified DPPH scavenging assay [10]. Different concentrations of A1, A2, and a standard antioxidant (BHA) were prepared. Following a 30-min incubation in the dark, the absorbance was measured at 517 nm. This measurement was used to determine the ability of each compound to neutralize DPPH radicals (Table 2). The antioxidant capacity of each compound was then expressed as an IC50 value (μg/mL), which represents the concentration needed to reduce DPPH activity by 50% (Figure 5). Additionally, the percentage of DPPH radical inhibition for each sample was calculated using the following formula:
where:
% Inhibition = [(A control − A sample)/A control] × 100

Table 2.
Table of Antioxidant Evaluation.
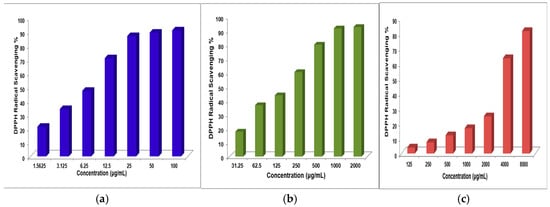
Figure 5.
(a) DPPH radical scavenging activities of various concentrations of BHA standard; (b) DPPH radical scavenging activities of various concentrations of A1; (c) DPPH radical scavenging activities of various concentrations of A2.
A control: Absorbance of the DPPH solution in methanol only.
A sample: Absorbance of the tested product or standard.
4. Conclusions
New Hydrazine carboximidamides were successfully synthesized with excellent yields using a simple condensation reaction. These compounds demonstrated effective inhibition of tubulin and AChE in molecular docking studies. Additionally, the ADMET analysis indicates that these compounds can cross the blood-brain barrier (BBB), suggesting their potential as anti-Alzheimer’s agents. Furthermore, they have proven to be antioxidant compounds, especially A1, with an IC50 of 168.06 ± 2.92 µg/mL.
Author Contributions
Conceptualization, A.A.B. and S.R.; methodology, A.A.B.; software, A.A.B.; validation, A.A.B.; formal analysis, R.D.; investigation, A.A.B.; resources, A.A.B.; data curation, A.A.B.; writing—original draft preparation, A.A.B.; writing—review and editing, A.A.B.; visualization, A.A.B.; supervision, A.A.B.; project administration, S.R.; funding acquisition, S.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Verma, S.; Lal, S.; Narang, R.; Sudhakar, K. Quinoline Hydrazide/Hydrazone Derivatives: Recent Insights on Antibacterial Activity and Mechanism of Action. ChemMedChem 2023, 18, e202200571. [Google Scholar] [CrossRef]
- Fan, M.; Yang, W.; Liu, L.; Peng, Z.; He, Y.; Wang, G. Design, synthesis, biological evaluation, and docking study of chromone-based phenylhydrazone and benzoylhydrazone derivatives as antidiabetic agents targeting α-glucosidase. Bioorg. Chem. 2023, 132, 106384. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Ji, R.; Wang, H.; Li, S.; Zhang, G. Discovery of Novel α-Aminophosphonates with Hydrazone as Potential Antiviral Agents Combined With Active Fragment and Molecular Docking. Front. Chem. 2022, 10, 911453. [Google Scholar] [CrossRef] [PubMed]
- Katariya, K.D.; Shah, S.R.; Reddy, D. Anticancer, antimicrobial activities of quinoline based hydrazone analogues: Synthesis, characterization and molecular docking. Bioorg. Chem. 2020, 94, 103406. [Google Scholar] [CrossRef] [PubMed]
- Şenkardeş, S.; İhsan Han, M.; Gürboğa, M.; Özakpinar, Ö.B.; Küçükgüzel, Ş.G. Synthesis and anticancer activity of novel hydrazone linkage-based aryl sulfonate derivatives as apoptosis inducers. Med. Chem. Res. 2022, 31, 368–379. [Google Scholar] [CrossRef]
- Baier, A.; Kokel, A.; Horton, W.; Gizińska, E.; Pandey, G.; Szyszka, R.; Török, B.; Török, M. Organofluorine Hydrazone Derivatives as Multifunctional Anti-Alzheimer’s Agents with CK2 Inhibitory and Antioxidant Features. ChemMedChem 2021, 16, 1927–1932. [Google Scholar] [CrossRef] [PubMed]
- Belkheiri, N.; Bouguerne, B.; Bedos-Belval, F.; Duran, H.; Bernis, C.; Salvayre, R.; Nègre-Salvayre, A.; Baltas, M. Synthesis and antioxidant activity evaluation of a syringic hydrazones family. Eur. J. Med. Chem. 2010, 45, 3019–3026. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.S.; Ge, Y.X.; Cheng, Z.Q.; Song, J.L.; Wang, Y.Y.; Zhu, K.; Zhang, H. Discovery of new multifunctional selective acetylcholinesterase inhibitors: Structure-based virtual screening and biological evaluation. J. Comput. Aided Mol. Des. 2019, 33, 521–530. [Google Scholar] [CrossRef] [PubMed]
- Sana, S.; Reddy, V.G.; Reddy, T.S.; Tokala, R.; Kumar, R.; Bhargava, S.K.; Shankaraiah, N. Cinnamide derived pyrimidine-benzimidazole hybrids as tubulin inhibitors: Synthesis, in silico and cell growth inhibition studies. Bioorg. Chem. 2021, 110, 104765. [Google Scholar] [CrossRef] [PubMed]
- Blois, M.S. Antioxidant determinations by the use of a stable Free Radical. Nature 1958, 4617, 1119–1200. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).